Radiolabeled biomolecules targeted at tumor-specific enzymes, receptors, and transporters in cancer cells represent an intensively investigated and promising class of molecular tools for the cancer diagnosis and therapy. High specificity of such biomolecules is a prerequisite for the treatment with a lower burden to normal cells and for the effective and targeted imaging and diagnosis. The most impressive outputs in categories of newly developed structures, as well as imaging and diagnosis approaches, and the most intensively studied oncological diseases in this context, are emphasized in order to show future perspectives of radiometal labeled amino acid-based compounds in nuclear medicine.
- Amino acid
- peptide
- bifunctional chelating agent (BFCA)
- cancer
- receptor
- imaging
1. Introduction
The great emphasis in nuclear medicine is put on a synthesis and study of radiolabeled amino acid-derived biomolecules with a selective distribution and binding to target structures in living cells and tissues, i.e., enzymes, transporters, or peptide receptors. This allows targeted therapy and diagnostic evaluation of pathological changes in many fields, such as oncology, neurology, endocrinology, cardiology, and also investigation of inflammation processes or infection. Especially, malignant tumor diseases are of the biggest interest because of their increasing global incidence, and placing second in the causes of death.
Target-specific radiolabeled compounds, which bind with high affinity to specific protein structures (e.g., active places in enzymes or receptors), represent effective probes in a recognizing and visualizing tumor cells in their early stage. All types of malignant solid tumors often exhibit lower oxygenation levels than their original tissues resulting in a hypoxic state. Hence, there is an urgent need to enhance detection approaches for a monitoring of various tumor types, including hypoxic cancer lesions. In this field, amino acid-based target-specific radiopharmaceuticals have become significant tools in modern oncology allowing cancer imaging on molecular and cellular level.[1]
The aim of this entry is to summarize the most significant outputs related to the development of target-specific radiometal labeled biomolecules for imaging of severe tumor types and tumors with an increased incidence, including the most employed bifunctional chelating agents and peptide families and receptors such as somatostatin, cholecystokinin/gastrin, bombesin, integrins, and hypoxia endogenous markers, as well as inhibitors of prostate-specific membrane antigen and fibroblast activation protein.
2. Metal Radionuclides and Chelators Currently Used in Nuclear Medicine
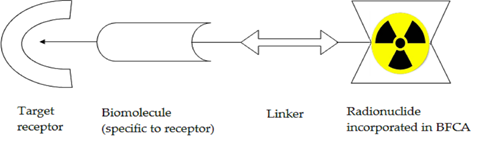
Radiometallic compounds with targeted biodistribution and binding in the human body (i.e., target-specific) include in their structure: (i) biomolecules as a crucial biodistribution component (specific to receptor); (ii) a linker as a connecting component preserving specificity of biomolecule when attaching; (iii) a bifunctional chelating agent (BFCA); and (iv) metal radionuclides (see Figure 1).
Figure 1. Basic scheme of a potential target-specific radiopharmaceutical.
2.1. Metal Radionuclides
Metal radionuclides belong to the most powerful and the most employed labels in nuclear medicine. Nuclear medicine research is currently focused on development of a highly potent target-specific biomolecule labeled with positron emitters, predominantly gallium-68, but also copper-64 and others. Apparently, other potential radionuclides such as zirconium-89, yttrium-86, and cobalt-55 have been included in recent studies. Anyway, there is still a leading position of technetium-99m in diagnostic clinical practice. In research, a prognosis for the development of Tc-radiopharmaceuticals is also quite positive due to novel modifications of BFCA and linkers continuously presented and developed for SPECT imaging. Basic parameters of the most common metallic radionuclides for diagnostic nuclear medicine are summarized in Table 1.
Table 1. Selected metallic radionuclides employed in diagnostic nuclear medicine.
|
Isotope |
Radiation Type |
Emax [keV] (Decay %) |
Half-life |
Production (Common Reaction) |
|
99mTc |
γ |
141 (89.1%) |
6.01 h |
99Mo/99mTc generator (cyclotron alternatively) |
|
111In |
171.3 (90.2%) 245.4 (94%) |
2.83 d |
cyclotron, 112Cd(p, 2n)111In |
|
|
67Ga |
93.3 (37%), 184.6 (20.4%), 300.2 (16.6%) |
3.26 d |
cyclotron, 68Zn(p, 2n)67Ga |
|
|
64Cu |
β+ |
653 (17.6%) |
12.7 h |
cyclotron, 64Ni(p, n)64Cu |
|
68Ga |
836 (89%) |
67.7 m |
68Ge/68Ga generator (cyclotron alternatively) |
|
|
89Zr |
|
395 (23%) |
3.3 d |
cyclotron, 89Y(p, n)89Zr |
2.2. Bifunctional Chelating Agents (BFCA)
Since the metallic radionuclides themselves cannot be utilized in a direct radiolabeling of amino acid-based target-specific compounds (peptides, proteins), it is necessary to develop bifunctional chelating agents (BFCA) [2]. An appropriate BFCA can properly attach both a metallic radionuclide and a biomolecule. The double function of BFCA helps the biomolecule to retain its receptor specificity and to match metal properties with the intended utilization in the imaging / therapy of various diseases.
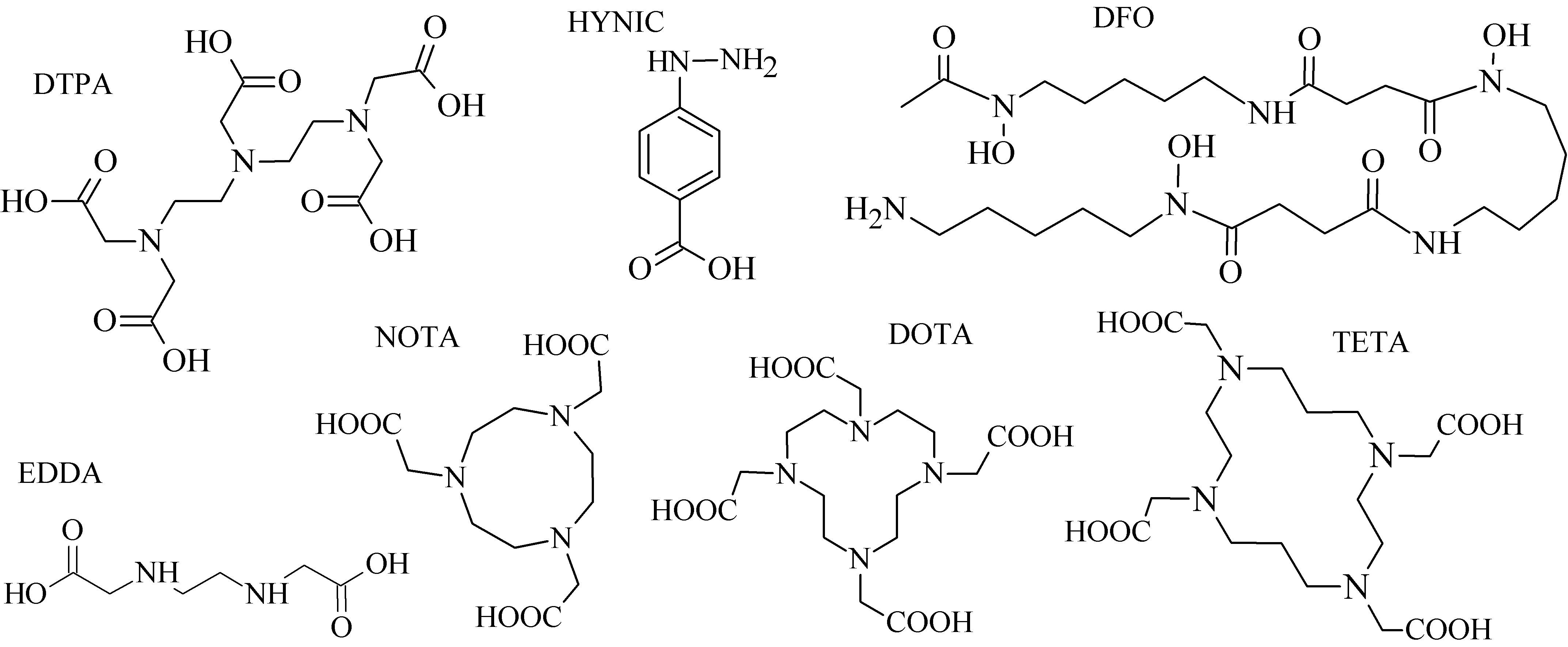
Various acyclic and cyclic BFCA have been introduced into (potential) radiopharmaceuticals. Traditional examples of acyclic and cyclic BFCA are discussed in this Section (for selected representatives see Figure 2), while the newer developed BFCA in Sections 3.1.-3.2.
From a group of acyclic BFCA, the polyaminopolycarboxylic acids-derived molecules, such as DTPA, EDDA, EDTA, as well as tripeptide MAG3, are the most commonly used acyclic BFCA containing hard donor atoms (N,O) in their molecule to form the coordination bond with metallic radionuclide. Another acyclic chelator, a siderophore-based desferrioxamine-B (DFO) has been utilized for effective radiolabeling of biomolecules with a metal. A significant advantage of the acyclic BFCA is faster metal binding kinetics, resulting in a faster radiolabeling procedure [3]. On the contrary, acyclic BFCA form less stable complexes than cyclic ones due to a higher interaction probability and more fixed geometry of donor atoms in the cyclic BFCA [4]. The cyclic BFCA are beneficial generally by providing more kinetically inert and thermodynamically stable complexes with metal radionuclides.
The cyclic BFCA containing macrocycle such as DOTA, NOTA, TETA, and their derivatives as well as various structurally related analogues are holding an important position in syntheses of radiolabeled peptide-based compounds over a long period. Abrams and co-workers used 6-hydrazinopyridin-3-carboxylic acid, in short HYNIC, for radiolabeling of a polyclonal antibody with technetium-99m [5]. Ever since, HYNIC has become the most convenient chelator for 99mTc-labeled peptides and antibodies. Other chelators related to bisthiosemicarbazone [6,7][6][7], cyclam [8,9][8][9], and sarcophagine [10,11]10][11] have been increasingly studied to improve kinetic inertness and stability of complexes, especially those with copper isotopes. Several new next generation cyclic chelators or chelators derived from traditional ones with improved properties have been developed over past decade such as PCTA, AAZTA, TRAP, THP, and fusarinine C [12] [12].
3. Radiolabeling Approaches for Target-Specific Peptide Molecules
Radiolabeled peptides as amino acid-based biomolecules are in the center of interest in the field of nuclear medicine and pharmacy because their biological action is mediated upon selective binding to specific peptide receptors and transporters overexpressed in numerous tumor cells. These receptors have shown potential as a molecular target for tumor imaging or targeted therapy with radiolabeled peptides (for the most important onco-specific peptide receptors and radiolabeled peptides see Section 4). The following Subsections (3.1.–3.3.) are dealing with current radiolabeling approaches used for peptides.
3.1. Conventional Radiolabeling Approaches of Peptides with Metallic Radionuclides
The choice of a radiolabeling approach depends on radionuclide nature and a bioactive molecule. Since a direct approach is more difficult to be used for a metal attachment to biomolecules and provides low site-specific and unstable products [13], an indirect labeling method with BFCA has become preferred for a metal-peptide linkage.
Indirect labeling approaches, such as pre-labeling (metal labeling before conjugation with biomolecule) or post-labeling (metal labeling after conjugation with biomolecule), are of the routine for 99mTc-coordination. The pre-labeling procedure is very useful in research to prove the concept and define the chemistry, contrary to a clinical use because of a long lasting radiosynthesis and hardly accomplished kit formulation [14]. In past few years, [99mTc]Tc-HYNIC, DTPA and MAG3 have been the most commonly used core for the conventional radiolabeling of bioactive peptides for tumor imaging.
For a 68Ga-labeling procedure, nitrogen and oxygen donor groups of carboxylates, hydroxamates, amines are coordinated [3]. Well-known representatives and the most frequently used BFCA are derived from 1,4,7-triazacyclononane and 1,4,7,10-tetraazacyclododecane, e.g., DOTA and NOTA, including their recently developed derivatives such as TRAP, PCTA, NOTP, and THP and DATA.
Indium-111 has several properties for coordination chemistry with gallium-68 in common. Softer donor groups can be offered to create seven or eight-coordinated complexes [49]. The DTPA- and DOTA-based chelators usually in t-butyl forms are generally the most employed for the 111In-labeling [15].
The design of copper radiopharmaceuticals has put emphasis on polyaza-macrocycles derived BFCA such as cyclam, sarcophagines, tiosemicarbazones. Due to only moderate stability of [64Cu]Cu-DOTA-labeled biomolecules under in vivo conditions and high liver accumulation, a number of cross-bridged cyclam derivatives were developed to form more stable 64Cu-complexes [8,9,16][16].
Zirconium prefers anionic oxygen donor groups to create complexes with high coordination number [17]. In order to effectively utilize zirconium-89, various chelators have been employed such as DOTA, DTPA, as well as the most successful desferrioxamine B and 3-hydroxypyridin-2-one derivatives.
3.2. Radiolabeling Approaches of Peptides with Metallic Radionuclide Based on Click-Chemistry
There are two main characteristics making the click chemistry attractive, i.e., the bioorthogonality of reactions and mild reaction conditions (usually at room temperature and in aqueous media) [18]. Additional benefits include the selectivity, rapidity, and modularity of click ligations. Mindt et al. developed and extended the “click-to-chelate” methodology for radiometallic ligation [19,20][19][20].
The most commonly used term in click chemistry is copper-catalyzed azide-alkyne cycloaddition (CuAAC, Figure 3A). In recent years, several catalyst-free site-specific reactions have been investigated for effective radiolabeling of peptide biomolecules and nanomaterials including tetrazines and trans-alkenes for the inverse electron-demand Diels–Alder reaction (IEDDA), azide and cyclooctyne functionalities for the strain-promoted azide-alkyne cycloaddition (SPAAC), and functionalized phosphanes for the Staudinger ligation (Figure 3B–D) [21-23][21][22][23].
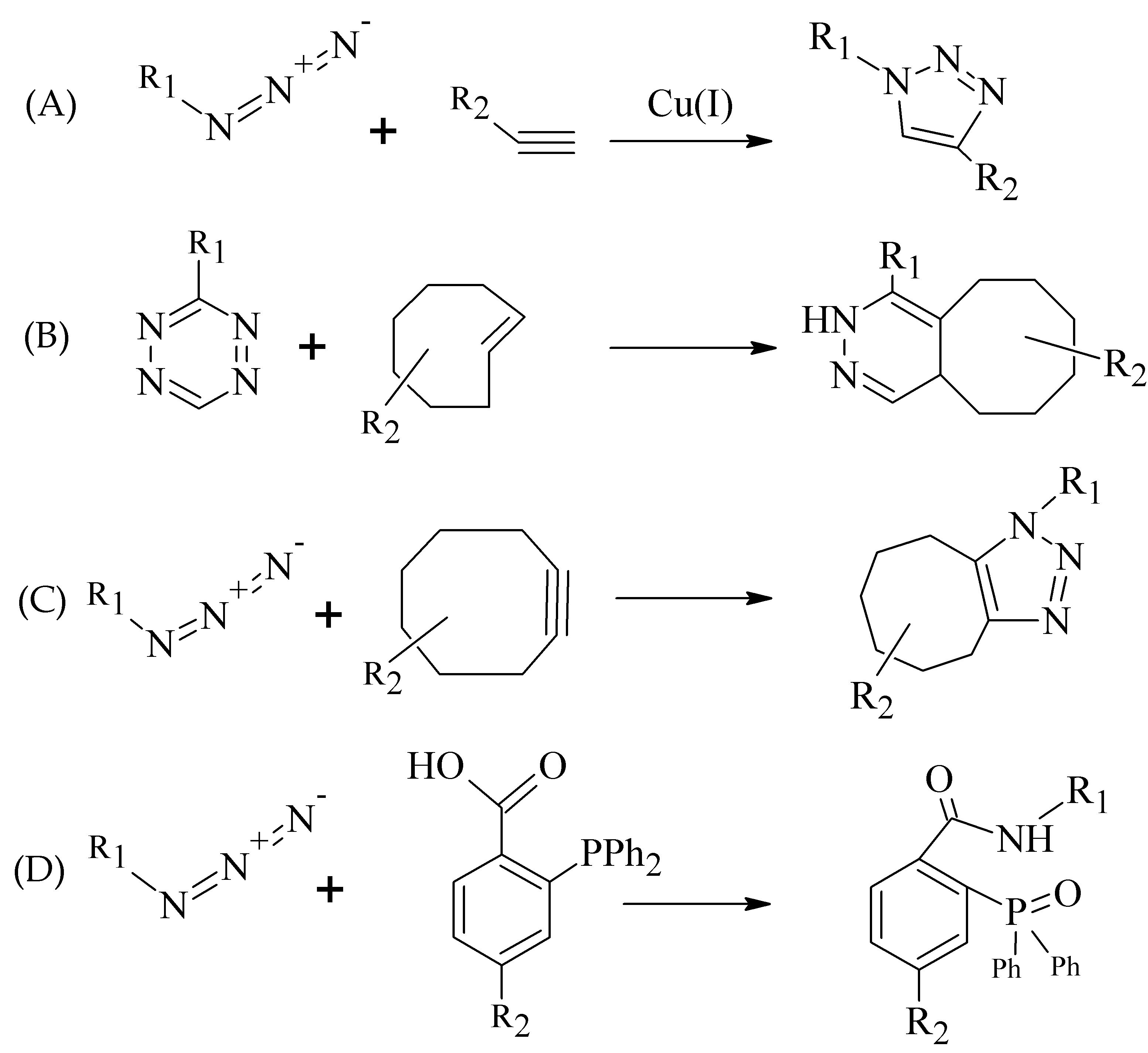
Within the “click-to-chelate” methodology, the development of new clickable chelators is currently attracting a growing interest. New clickable chelators have been designed for 99mTc-labeled peptides, as well as for 68Ga- and 64Cu-labeled probes to obtain an increased hydrophilicity and decreased hepatobiliary retention (see examples in Figure 4).
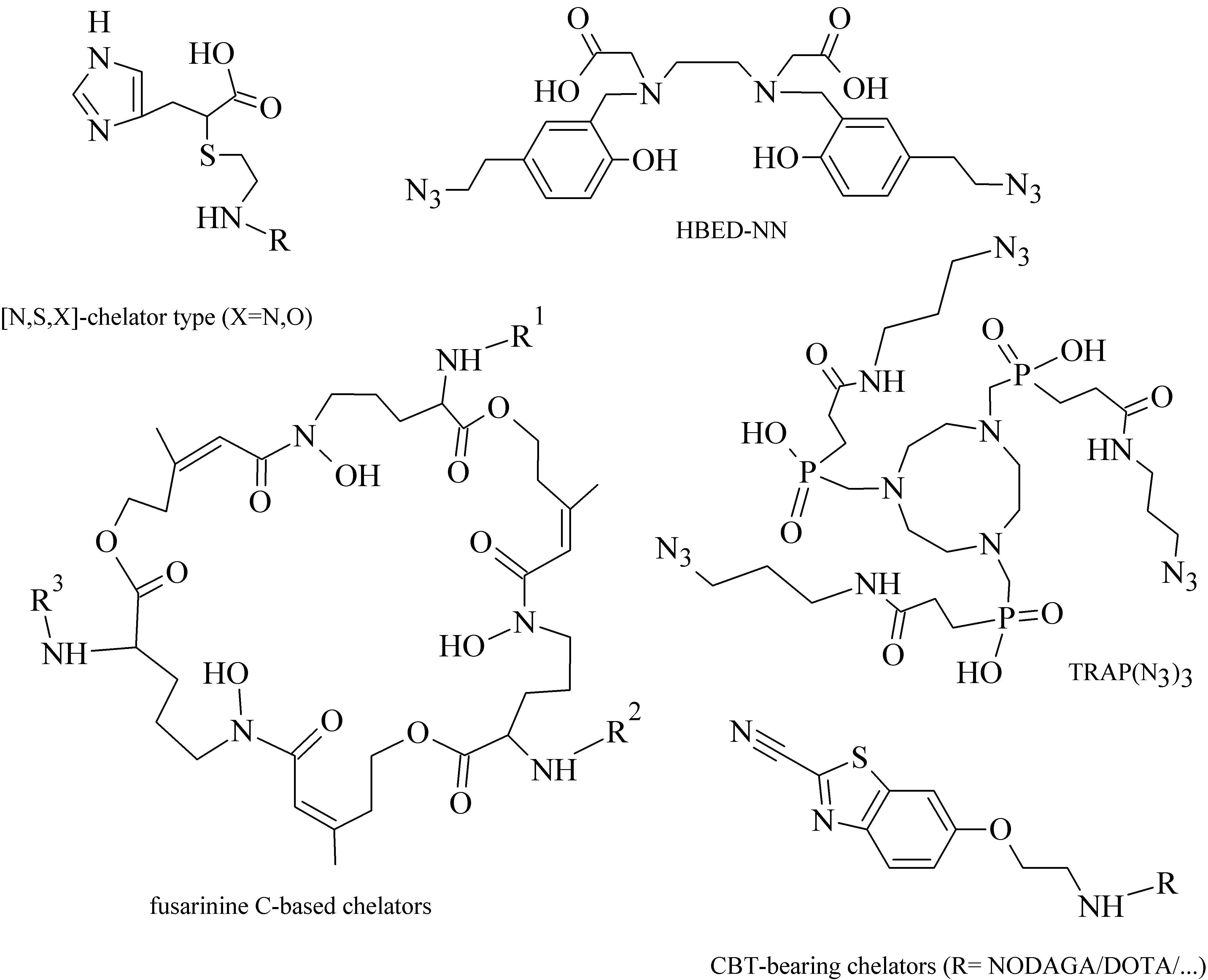
3.3. Radiolabeling Approaches of Peptides with Metallic Radionuclide Based on Nanoparticles
Over past 10 years, tens of articles have been focused on the metal-labeled nanoparticles (NP) conjugated to various peptides for SPECT and PET cancer imaging (see a representative image of radiolabeled nanoparticles using electron microscopy in Figure 5).
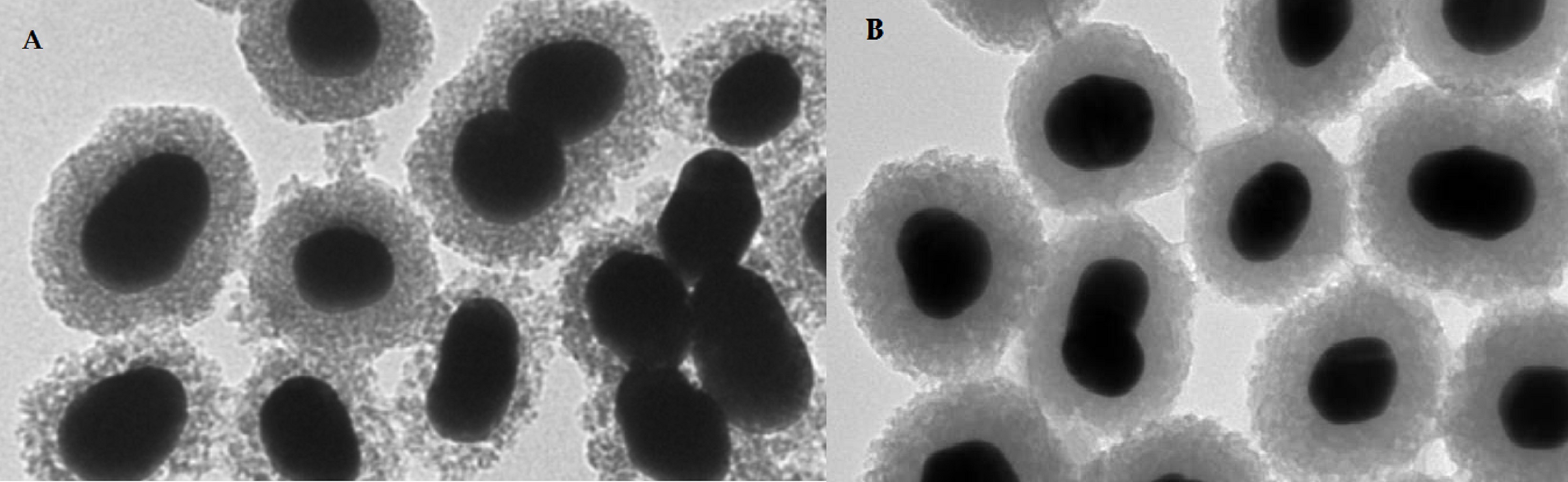
An indirect method has been much more preferred for the radiolabeling of NP with metals. In the indirect method, BFCA is necessary to allow a stable linkage between radionuclide and NP [30]. Gold NPs have been conjugated to peptides with [99mTc]Tc-HYNIC for integrin-positive glioma imaging [31], with [99mTc]Tc-DTPA for breast cancer imaging [32], for gastrin releasing peptide receptor imaging [33,34][33][34] and somatostatin receptor-positive neuroendocrine tumor imaging [35]. The 64Cu- and 68Ga-labeled NPs functionalized with a peptide were reported in several papers too. The multifunctional gold nanorod nanocarriers were bound with doxorubicin and conjugated to [64Cu]Cu-NOTA-RGD [36]; [64Cu]Cu-sulphide NPs conjugated to the pegylated bombesin [37]; [68Ga]Ga-DOTA-somatostatin and neurotensin analogues to gold NPs [38]; [68Ga]Ga-NODAGA-bombesin to the polyethylene glycol-coated ultra-small superparamagnetic iron-oxide nanoparticles [39]; and [68Ga]Ga-DOTA-bombesin analogue conjugated to the N,N,N-trimethyl chitosan-coated magnetic nanoparticles [40].
4. Onco-receptors and Their Radiometal Labeled Peptides for Tumor Imaging
In this Section, the most commonly studied onco-receptors are summarized, showing significant radiometal labeled peptide ligands and inhibitors of tumor-related proteins in clinical trials for tumor imaging. An illustrative example of a study [41] of radiolabeled [68Ga]Ga-OPS202 and [68Ga]Ga-DOTATOC biomolecules for neuroendocrine tumors imaging is in Figure 6.
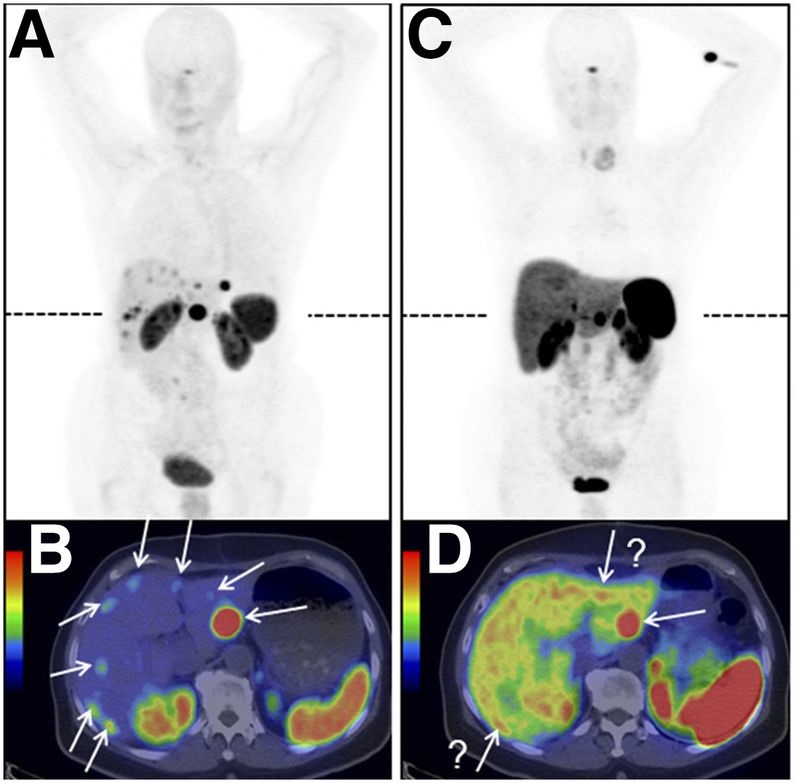
Many peptide compounds with high affinity to their receptors as molecular targets have been investigated over years including somatostatin and its analogues for somatostatin receptors (SSTR) imaging, bombesin (BBN) and its analogues for gastrin-releasing peptide receptor (GRPR) imaging, cholecystokinin and its analogues for cholecystokinin receptor (CCKR) imaging, exendin analogues for glucagon-like peptide 1 (GLP-1) receptor imaging, RGD analogues for αvβ3 integrins imaging, and other important peptide analogues, i.e. neurotensin, α-melanocyte stimulating hormone (α-MSH), substance P, and vasoactive intestinal peptide analogues.
Table 2. The amino acid sequences of the most commonly studied peptide ligands for onco-receptors.
|
Peptide |
Amino acid sequence |
|
somatostatin |
Ala-Gly-Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-Ser-Cys (3-14) disulfid |
|
bombesin |
Glp-Gln-Arg-Leu-Gly-Asn-Gln-Trp-Ala-Val-Gly-His-Leu-Met-NH2 |
|
cholecystokinin |
Asp-Tyr-Met-Gly-Trp-Met-Asp-Phe-NH2 |
|
glucagon-like peptide 1 |
His-Asp-Glu-Phe-Glu-Arg-His-Ala-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Val-Ser-Ser-Tyr-Leu-Glu-Gly-Gln-Ala-Ala-Lys-Glu-Phe-Ile-Ala-Trp-Leu-Val-Lys-Gly-Arg-NH2 |
|
RGD |
Arg-Gly-Asp |
|
neurotensin |
Glp-Leu-Tyr-Glu-Asn-Lys-Pro-Arg-Arg-Pro-Tyr-Ile-Leu-NH2 |
|
α-MSH |
Ac-Ser-Tyr-Ser-Met-Glu-His-Phe-Arg-Trp-Gly-Lys-Pro-Val-NH2 |
|
substance P |
Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH2 |
|
vasoactive intestinal peptide |
His-Ser-Asp-Ala-Val-Phe-Thr-Asp-Asn-Tyr-Thr-Arg-Leu-Arg-Lys-Gln-Met-Ala-Val-Lys-Lys-Tyr-Leu-Asn-Ser-Ile-Leu-Asn-NH2 |
The peptide receptors regulate various endocrine and exocrine secretion throughout a human body. Under pathological conditions, peptide receptors are overexpressed in many tumor types such as SSTR in GEP-NET, pituitary adenomas, breast cancer, small-cell lung cancer, melanoma [42-46][42][43][44][45][46]; GRPR in breast, lung, pancreas, colon, and prostate [47-49][47][48][49]; CCK2R in small cell lung cancers and medullary thyroid carcinomas [50]; GLP-1R in insulinomas, gastrinoma, pulmonary NET, and medullary thyroid cancer [51,52][51][52]; and αvβ3 integrins associated with angiogenesis, tumor growth, invasion, and metastasis in breast, non-small cell lung, pancreatic, ovarian, and prostate cancer, oral squamous cell carcinoma, or glioma [53]. Another common feature for tumor development and progression is hypoxia, a phenomenon when a level of oxygen is below its demands. Hypoxia is a key component in cellular expression, tumor blood vessel formation, cancer progression, metastasis, often leading to cell death. Many studies have been comprised of radiolabeled small nitroimidazole derivatives [54-57][54][55][56][57], small sulfonamide- and peptide-based biomolecules [28,58-60][58][59][60] and monoclonal antibodies [61[61][62],62], resulting in a development of new agents capable of accessing to overexpressed proteins under hypoxic state (i.e., hypoxia inducible factor HIF-1 regulated genes for carbonic anhydrase CA IX, vascular endothelial growth factor, angiopoietin-2, etc. [63]). A specific CA-binding of various 1,3,5-triazinyl-sulfonamide derivatives with amino-acid substituents has been demonstrated in our several recent works [64].
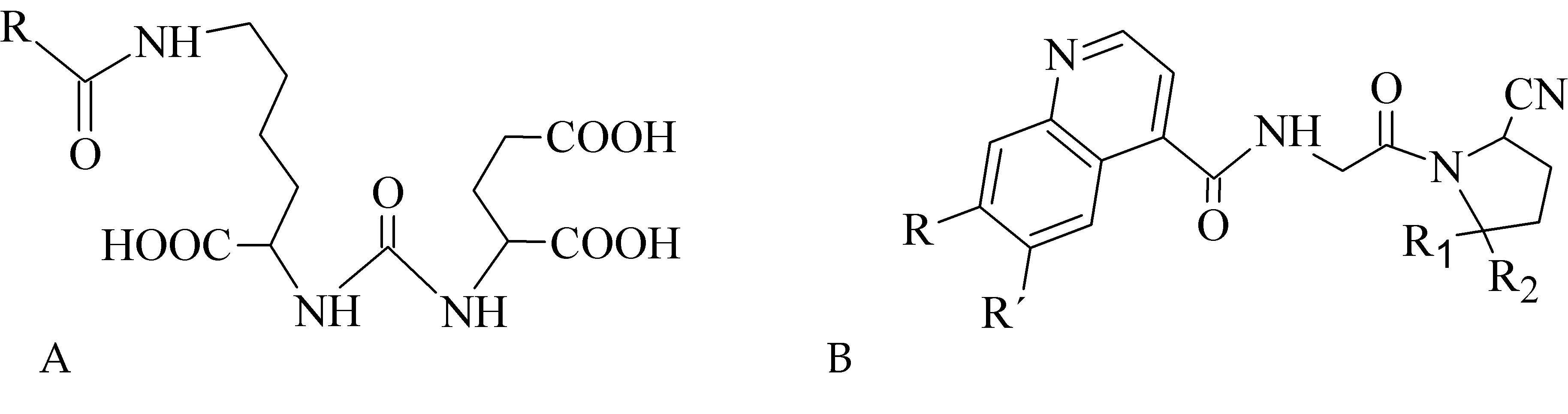
In recent years, inhibitors of cancer-related proteins based on small peptide biomolecules are widely developed and investigated such as prostate-specific membrane antigen (PSMA) and fibroblast activation protein (FAP) inhibitors. The PSMA is primarily expressed in benign and malignant prostatic tissue [65], but also in other tumor types including breast, gastric, and colorectal cancer, lung and renal carcinoma, and brain tumors [66-71][66][67][68][69][70][71]. The FAP overexpression has been observed in various malignancies, e.g., pancreatic, hepatocellular, lung, breast, colorectal, or ovarian [72-77][72][73][74][75][76][77]. Various radiolabeled small peptide-based inhibitors containing Glu-C(O)-Lys (EuK) sequence (Figure 7A) for PSMA targeting and FAP-inhibitors (FAPI) based on 2-cyanopyrrolidin-quinoline carboxamide (Figure 7B) have been recently developed to effectively localize and treat related tumors.
Figure 7. Structural motifs of small peptide inhibitors of proteins. (A) EuK motif as a base for PSMA inhibitors, (B) 2-cyanopyrrolidin-quinoline carboxamides as a base for FAP inhibitors.
Table 3. Summary of the most significant radiolabeled peptide analogues in recent clinical trials for receptor-positive tumor imaging.
|
Composition of Studied Compounds - Metal Radionuclide - BFCA - Linker - Peptide |
Results and Findings - Patients in Clinical Trials - Onco-receptor / Cancer Type Studied - Imaging Technique Used - Benefits/Limitations/Conclusion |
Reference |
|
- 68Ga - DOTA, NODAGA - x - JR11, TOC |
- 12 patients - somatostatin rec. / GEP-NET - PET/CT - very high TBR and image contrast of liver lesions for [68Ga]Ga-NODAGA-JR11; studies in larger patient group proven |
[41] |
|
- 68Ga - DATA - x - TOC |
- 53 patients - somatostatin rec. / GEP-NET - PET/CT - comparable imaging profile of [68Ga]Ga-DATA-TOC with DOTA-NOC; DATA-conjugate useful for instant kit labeling |
[78] |
|
- 68Ga - DOTA -4-amino-1-carboxymethylpiperidine - RM2 |
- 16 patients - gastrin-releasing peptide rec. / prostate - PET/CT, multiparametric MRI - fusion of MRI and PET/CT improved detection of a primary disease, but expression of GRPR and PSMA was not correlated |
[79] |
|
- 111In - DOTA - x - (D-Glu1-6)minigastrin |
- 16 patients - cholecystokinin rec. / advanced medullary thyroid - SPECT/CT - high uptake in lesions and favorable dosimetry confirmed, but increased calcitonin concentrations in blood; initiation of 177Lu-analogue assessment |
[80] |
|
- 99mTc - HYNIC, tricine, TPPTS - x - RGD2 |
- 20 patients - αvβ3 integrins / breast - gamma camera - good uptake in breast lesions and also metastatic sites in lymph nodes visible in 2 patients - useful easily available kit for further clinical studies |
[81] |
|
- 68Ga - THP - Ahx - EuK motif |
- 118 patients - PSMA / prostate - PET/CT - PET/CT impacts on management decisions in high-risk prostate cancer prior to radical therapy and biochemical recurrence |
[82] |
|
- 68Ga, 177Lu - DOTA - piperazine - FAPI-02,-04 |
- 23 patients together - fibroblast activation protein / fibrosarcoma, pancreatic, breast, lung, colon, thyroid, head and neck - microPET, PET/CT - [68Ga]Ga-FAPI-02 with TBR equal to or even better than [18F]FDG, PET/CT with 68Ga-probes can be performed without fasting and resting time |
[83-85] |
|
- 68Ga - DOTA - piperazine - FAPI-04 |
- 80 patients - fibroblast activation protein / 28 different tumor entities - PET/CT - the highest uptake in breast, esophagus, lung, pancreatic, head-neck, and colorectal cancer; FAPI limitations similar to those of FDG for renal and thyroid cancer |
[86] |
|
- 68Ga - DOTA - piperazine - FAPI-04 |
- 68/75 patients - fibroblast activation protein / different tumor types - PET/CT - higher TBR of FAPI compared to FDG for brain metastases, FAPI identified more lesions for hepatic and peritoneal tumor manifestations, and had higher sensitivity in a detection of lymphonodal, osseous and visceral metastases |
[87,88] |
5. Concluding Remarks and Future Perspectives
In this entry, recent advances in the radiolabeling process of amino-acid based biomolecules, the most commonly used metal radionuclides, their chemistry and BFCA, as well as the most important peptide receptor families (including currently the most perspective field of PSMA and FAP ligands), were discussed. Continual efforts in proposing new structures with improved pharmacokinetic properties for selective targeting of cancer cells and effective utilization in imaging techniques should be guaranteed. The disease imaging on a molecular level, as well as radionuclide availability on-site, lower radiation burden, detection of early stage problem, and monitoring of a response to treatment in the combination with targeted therapy for a personalized approach to a patient, have a great potential to bring additional valuable outputs in the field of nuclear medicine in future.
Over the past years, great progress in a radiolabeling with metallic radionuclides has been demonstrated. Optimized structures of some of the newly developed radiolabeled biomolecules should provide enhanced affinity and selectivity to the onco-receptors, lower radiation dosage for patient, decreased interactions with other drugs or physiological proteins, without misrepresenting results, and, by that, a more favorable utilization in diagnostic nuclear medicine over other imaging techniques (e.g., MRI, CT). Current research is directed towards peptide radiolabeled agents that are aimed at proteins overexpressed in pancreatic, colorectal, prostate, and brain tumors. These types belong to the most frequently diagnosed and the most severe cancers. The integrin αvβ3 receptors from traditional receptor families and PSMA, as well as FAP ligands are very attractive and perspective probes due to their intense association and overexpression within a variety of cancer cells and new vasculature in general, and so tumor growth, proliferation, and metastasis.
Abbreviations
AAZTA 6-amino-6-methylperhydro-1,4-diazepinetetraacetic acid
AHX 6-aminohexanoic acid
CA carbonic anhydrase
CBT 2-cyanobenzothiazole
c(RGDfK) cyclo(-Arg-Gly-Asp-d-Phe-Lys)
CT computed tomography
CuAAC Cu(I)-catalyzed azide-alkyne cycloaddition
DATA 6-amino-1,4-diazepine-triacetate
DFO desferrioxamine-B
DOTA 2,2’,2’’,2’’’-(1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrayl)tetraacetic acid
DOTANOC DOTA-Nal3-octreotide
DOTATATE DOTA-Tyr3-octreotate
DOTATOC DOTA-Tyr3-octreotide
DTPA diethylenetriamine pentaacetic acid
EDDA ethylenediamine diacetic acid
EDTA ethylenediamine tetraacetic acid
FAP(I) fibroblast activation protein (inhibitors)
GEP NET gastroenteropancreatic neuroendocrine tumors
GLP glucagon-like peptide
GRPR gastrin-releasing peptide receptor
HBED-CC N,N’-bis-[2-hydroxy-5-(carboxyethyl)benzyl]ethylenediamine-N,N’-diacetic acid
HYNIC 6-hydrazinopyridin-3-carboxylic acid
IEDDA inverse electron-demand Diels-Alder reaction
JR11 p-Cl-Phe-cyclo(d-Cys-Aph(Hor)-d-Aph(cbm)-Lys-Thr-Cys)d-Tyr-NH2
MAG3 mercaptoacetylglycylglycylglycine
MRI magnetic resonance imaging
NeoBOMB1 d-Phe-Gln-Trp-Ala-Val-Gly-His-NH-CH[CH2-CH(CH3)2]2
NET neuroendocrine tumors
NODAGA 1,4,7-triazacyclononane-1-glutaric acid-4,7-diacetic acid
NOTA 2,2’,2’’-(1,4,7-triazanonane-1,4,7-triyl)triacetic acid
NOTP 1,4,7-Triazacyclononane-1,4,7-tri(methylene phosphonic acid)
NP nanoparticle
PCTA 3,6,9,15-tetraazabicyclo [9.3.1]pentadeca-1(15),11,13-triene-3,6,9-triacetic acid
PEG polyethylene glycol
PET positron emission tomography
PSMA prostate-specific membrane antigen
PSMA-11 Glu-NH-CO-NH-Lys(Ahx)-HBED-CC
RGD arginine-glycine-aspartic acid
RM2 DOTA-4-amino-1-carboxymethylpiperidine-d-Phe-Gln-Trp-Ala-Val-Gly-His-Sta-Leu-NH2
SERRS surface-enhanced resonance Raman scattering
SPAAC strain-promoted azide-alkyne cycloaddition
SPECT single-photon emission computed tomography
SST(R) somatostatin (receptor)
TBR tumor-to-background ratio
TETA 2,2’,2’’,2’’’-(1,4,8,11-tetraazacyclotetradecane-1,4,8,11-tetrayl)tetraacetic acid
THP tris(hydroxypyridinone)
TOC Tyr3-octreotide
TPPTS trisodium triphenylphosphine-3,3′,3″-trisulfonate
TRAP 1,4,7-triazacyclononane-1,4,7-tris(methylene(2-carboxyethylphosphinic acid))
α-MSH α-melanocyte stimulating hormone
Author Contributions: M.B.M. conceptualization, writing of entry, editing; P.M. conceptualization, editing, and supervision. All authors have read and agreed to the published version of the manuscript.
Acknowledgements: This work was supported by the projects APVV-15-0585, VEGA 1/0463/18, KEGA 027UK-4/2020.
Conflicts of Interest: The authors declare no conflicts of interest.
References
- Okarvi, S. Peptide-based radiopharmaceuticals and cytotoxic conjugates. Cancer Treat. Rev. 2008, 34, 13–26, doi:10.1016/j.ctrv.2007.07.017.
- Brechbiel, M.W. Bifunctional Chelates for Metal Nuclides. J. Nucl. Med. Mol. Imaging 2008, 52, 166–173.
- Bartholoma, M. Recent Developments in the Design of Bifunctional Chelators for Metal-based Radiopharmaceuticals Used in Positron Emission Tomography. Chim. Acta 2012, 389, 36–51, doi:10.1016/j.ica.2012.01.061.
- Wängler, B.; Schirrmacher, R.; Bartenstein, P.; Wängler, C. Chelating Agents and their Use in Radiopharmaceutical Sciences. Mini Rev. Med. Chem. 2011, 11, 968–983, doi:10.2174/138955711797068445.
- Abrams, M.J.; Juweid, M.; Strauss, H.W.; Fischman, A.J. Technetium-99m-human polyclonal IgG radiolabeled via the hydrazino nicotinamide derivative for imaging focal sites of infection in rats. Nucl. Med. 1990, 31, 2022–2028.
- Dearling, J.L.J.; Blower, P.J. Redox-active metal complexes for imaging hypoxic tissues: Structure-activity relationships in copper(II) bis(thiosemicarbazone) complexes. Commun. 1998, 22, 2531–2532, doi:10.1039/A805957H.
- Blower, P.J.; Castle, T.C.; Cowley, A.R.; Went, M.J. Structural trends in copper(II) bis(thiosemicarbazone) radiopharmaceuticals. Dalton Trans. 2003, 23, 4416–4425, doi:10.1039/B307499D.
- Boswell, C.A.; Regino, C.A.S.; Baidoo, K.E.; Brechbiel, M.W. Synthesis of a cross-bridged cyclam derivative for peptide conjugation and 64Cu radiolabeling. Chem. 2008, 19, 1476–1484, doi:10.1021/bc800039e.
- Sun, X.; Wuest, M.; Weisman, G.R.; Anderson, C.J. Radiolabeling and in vivo behavior of copper-64-labeled cross-bridged cyclam ligands. Med. Chem. 2002, 45, 469–477, doi:10.1021/jm0103817.
- Tan, K.V.; Pellegrini, P.A.; Skelton, B.W.; Barnard, P.J. Triamidetriamine bearing macrobicyclic and macrotricyclic ligands: Potential applications in the development of copper-64 radiopharmaceuticals. Chem. 2014, 53, 468–477, doi:10.1021/ic4024508.
- Cai, H.; Fissekis, J.; Conti, P.S. Synthesis of a novel bifunctional chelator AmBaSar based on sarcophagine for peptide conjugation and 64Cu radiolabelling. Dalton Trans. 2009, 5395–5400, doi:10.1039/B902210D.
- Tsionou, M.I.; Knapp, C.E.; Foley, C.A; Ma, M.T. Comparison of macrocyclic and acyclic chelators for gallium-68 radiolabelling. RSC Adv. 2017, 7, 49586–49599, doi:10.1039/C7RA09076E.
- Fani, M.; Mäcke, H.R. Radiopharmaceutical development of radiolabelled peptides. J. Nucl. Med. Mol. Imaging 2012, 39, 11–30, doi:10.1007/s00259-011-2001-z.
- Liu, S. Bifunctional coupling agents for radiolabeling of biomolecules and target-specific delivery of metallic radionuclides. Drug Deliv. Rev. 2008, 60, 1347–1370, doi:10.1016/j.addr.2008.04.006.
- Heppeler, A.; Froidevaux, S.; Eberle, A.N.; Mäcke, H.R. Receptor Targeting for Tumor Localisation and Therapy with Radiopeptides. Med. Chem. 2000, 7, 971–994, doi:10.2174/0929867003374516.
- Dale, A.V.; An, G.I.; Pandya, D.N.; Yoo, J. Synthesis and Evaluation of New Generation Cross-Bridged Bifunctional Chelator for 64Cu Radiotracers. Chem. 2015, 54, 8177–8186, doi:10.1021/acs.inorgchem.5b01386.
- Deri, M.A.; Zeglis, B.M.; Francesconi, L.C.; Lewis, J.S. PET Imaging with 89Zr: From Radiochemistry to the Clinic. Med. Biol. 2013, 40, 3–14, doi:10.1016/j.nucmedbio.2012.08.004.
- Nwe, K.; Brechbiel, M.W. Growing Applications of “Click Chemistry” for Bioconjugation in Contemporary Biomedical Research. Cancer Biother. Radiopharm. 2009, 24, 289–302, doi:10.1089/cbr.2008.0626.
- Mindt, T.; Struthers, H.; Brans, L.; Schibli, R. “Click to Chelate”: Synthesis and Installation of Metal Chelates into Biomolecules in a Single Step. Am. Chem. Soc. 2006, 128, 15096–15097, doi:10.1021/ja066779f.
- Struthers, H.; Spingler, B.; Mindt, T.L.; Schibli, R. “Click-to-Chelate”: Design and Incorporation of Triazole-Containing Metal-Chelating Systems into Biomolecules of Diagnostic and Therapeutic Interest. Eur. J. 2008, 14, 6173–6183, doi:10.1002/chem.200702024.
- Meyer, J.P.; Adumeau, P.; Lewis, J.S.; Zeglis, B.M. Click Chemistry and Radiochemistry: The First 10 Years. Chem. 2016, 27, 2791–2807, doi:10.1021/acs.bioconjchem.6b00561.
- Mushtaq, S.; Yun, S.J.; Jeon, J. Recent Advances in Bioorthogonal Click Chemistry for Efficient Synthesis of Radiotracers and Radiopharmaceuticals. Molecules 2019, 24, 3567, doi:10.3390/molecules24193567.
- Svatunek, D.; Houszka, N.; Hamlin, T.A.; Bickelhaupt, F.M.; Mikula, H. Chemoselectivity of Tertiary Azides in Strain-Promoted Alkyne-Azide Cycloadditions. Eur. J. 2019, 25, 754–758, doi:10.1002/chem.201805215.
- Makarem, A.; Sarvestani, M.K.; Klika, K.D.; Kopka, K. A Multifunctional HBED-Type Chelator with Dual Conjugation Capabilities for Radiopharmaceutical Development. Synlett 2019, 30, doi:10.1055/s-0039-1690194.
- Baranyai, Z.; Reich, D.; Vagner, A.; Notni, J. A shortcut to high-affinity Ga-68 and Cu-64 radiopharmaceuticals: One-pot click chemistry trimerisation on the TRAP platform. Dalton Trans. 2015, 44, 11137–11146, doi:10.1039/C5DT00576K.
- Radford, L.L.; Papagiannopoulou, D.; Gallazzi, F.; Hennkens, H.M. Synthesis and evaluation of Re/99mTc(I) complexes bearing a somatostatin receptor-targeting antagonist and labeled via a novel [N,S,O] clickable bifunctional chelating agent. Med. Chem. 2019, 27, 492–501, doi:10.1016/j.bmc.2018.12.028.
- Summer, D.; Mayr, S.; Petrik, M.; Decristoforo, C. Pretargeted Imaging with Gallium-68—Improving the Binding Capability by Increasing the Number of Tetrazine Motifs. Pharmaceuticals 2018, 11, 102, doi:10.3390/ph11040102.
- Chen, K.T.; Nguyen, K.; Ieritano, C.; Gao, F.; Seimbille, Y. A Flexible Synthesis of 68Ga-Labeled Carbonic Anhydrase IX (CAIX)-Targeted Molecules via CBT/1,2-Aminothiol Click Reaction. Molecules 2019, 24, 23, doi:10.3390/molecules24010023.
- Wall, M.; Shäffer, T.M.; Harmsen, S.; Kircher, M. Chelator-Free Radiolabeling of SERRS Nanoparticles for Whole-Body PET and Intraoperative Raman Imaging. Theranostics 2017, 3068–3077, doi:10.7150/thno.18019.
- Varani, M.; Galli, F.; Auletta, S.; Signore, A. Radiolabelled nanoparticles for cancer diagnosis. Transl. Imaging 2018, 6, 271–292, doi:10.1007/s40336-018-0283-x.
- Morales-Avila, E.; Ferro-Flores, G.; Gomez-Olivan, L. Multimeric system of 99mTc-labeled gold nanoparticles conjugated to c[RGDfK(C)] for molecular imaging of tumor α(v)β(3) expression. Chem. 2011, 22, 913–922, doi:10.1021/bc100551s.
- Peiris, P.M.; Deb, P.; Doolittle, E.; Karathanasis, E. Vascular targeting of a gold nanoparticle to breast cancer metastasis. Pharm. Sci. 2015, 104, 2600–2610, doi:10.1002/jps.24518.
- Mendoza-Sanchez, A.N.; Ferro-Flores, G.; Camacho-Lopez, M.A. Lys3-bombesin conjugated to 99mTc-labelled gold nanoparticles for in vivo gastrin releasing peptide-receptor imaging. Biomed. Nanotechnol. 2010, 6, 375–384, doi:10.1166/jbn.2010.1132.
- Silva, F.; Gano, L.; Campello, M.P.C.; Kannan, R. In vitro/in vivo “peeling” of multilayered aminocarboxylate gold nanoparticles evidenced by a kinetically stable 99mTc-label. Dalton Trans. 2017, 46, 14572–14583, doi:10.1039/C7DT00864C.
- Orocio-Rodriguez, E.; Ferro-Flores, G.; Sanchez-Garcia, F.M. Two novel nanosized radiolabeled analogues of somatostatin for neuroendocrine tumor imaging. Nanosci. Nanotechnol. 2015, 15, 4159–4169, doi:10.1166/jnn.2015.9620.
- Xiao, Y.; Hong, H.; Matson, V.Z.; Gong, S. Gold Nanorods Conjugated with Doxorubicin and cRGD for Combined Anticancer Drug Delivery and PET Imaging. Theranostics 2012, 2, 757–768, doi:10.7150/thno.4756.
- Cai, H.; Xie, F.; Mulgaonkar, A.; Wu, C.; Ai, H. Bombesin functionalized 64Cu-copper sulfide nanoparticles for targeted imaging of orthotopic prostate cancer. Nanomedicine 2018, 13, 1695–1705, doi:10.2217/nnm-2018-0062.
- Chilug, L.E.; Leonte, R.A.; Patrascu, M.E.B.; Niculae, D. In vitro binding kinetics study of gold nanoparticles functionalized with 68Ga-DOTA conjugated peptides. Radioanal. Nucl. Chem. 2017, 311, 1485–1493, doi:10.1007/s10967-016-5075-z.
- Lahooti, A.; Shanehsazzadeh, S.; Laurent, S. Preliminary studies of 68Ga-NODA-USPION-BBN as a dual-modality contrast agent for use in positron emission tomography/magnetic resonance imaging. Nanotechnology 2020, 31, 015102, doi:10.1088/1361-6528/ab4446.
- Hajiramezanali, M.; Atyabi, F.; Mosayebnia, M.; Beiki, D. 68Ga-radiolabeled bombesin-conjugated to trimethyl chitosan-coated superparamagnetic nanoparticles for molecular imaging: Preparation, characterization and biological evaluation. J. Nanomed. 2019, 14, 2591–2605, doi:10.2147/IJN.S195223.
- Nicolas, G.P.; Schreiter, N.; Kaul, F.; Wild, D. Sensitivity comparison of 68Ga-OPS202 and 68Ga-DOTATOC PET/CT in patients with gastroenteropancreatic neuroendocrine tumors: A prospective phase II imaging study. Nucl. Med. 2018, 59, 915–921, doi:10.2967/jnumed.117.199760.
- Cuccurullo, V.; Prisco, M.R.; Di Stasio, G.D.; Mansi, L. Nuclear Medicine in Patients with NET: Radiolabeled Somatostatin Analogues and their Brothers. Radiopharm. 2017, 10, 74–84, doi:10.2174/1874471010666170323115136.
- Cakir, M.; Dworakowska, D.; Grossman, A. Somatostatin receptor biology in neuroendocrine and pituitary tumours: Part 2—Clinical implications. Cell. Mol. Med. 2010, 14, 2585–2591, doi:10.1111/j.1582-4934.2010.01125_1.x.
- Orlando, C.; Raggi, C.C.; Bianchi, S.; Serio, M. Measurement of somatostatin receptor subtype 2 mRNA in breast cancer and corresponding normal tissue. Relat. Cancer 2004, 11, 323–332, doi:10.1677/erc.0.0110323.
- Lapa, C.; Hänscheid, H.; Wild, V.; Lückerath, K. Somatostatin receptor expression in small cell lung cancer as a prognostic marker and a target for peptide receptor radionuclide therapy. Oncotarget 2016, 7, 20033–20040, doi:10.18632/oncotarget.7706.
- Lum, S.S.; Fletcher, W.S.; O’Dorisio, M.S.; Caprara, M. Distribution and functional significance of somatostatin receptors in malignant melanoma. World J. Surg. 2001, 25, 407–412, doi:10.1007/s002680020102.
- Moreno, P.; Ramos-Alvarez, I.; Moody, T.W.; Jensen, R.T. Bombesin related peptides/receptors and their promising therapeutic roles in cancer imaging, targeting and treatment. Expert Opin. Ther. Targets 2016, 20, 1055–1073, doi:10.1517/14728222.2016.1164694.
- Baratto, L.; Jadvar, H.; Iagaru, A. Prostate Cancer Theranostics Targeting Gastrin-Releasing Peptide Receptors. Imaging Biol. 2017, 20, 501–509, doi:10.1007/s11307-017-1151-1.
- Nock, B.A.; Kaloudi, A.; Lymperis, E.; Maina, T.; Baum, R.P. Theranostic Perspectives in Prostate Cancer with the Gastrin-Releasing Peptide Receptor Antagonist NeoBOMB1: Preclinical and First Clinical Results. Nucl. Med. 2017, 58, 75–80, doi:10.2967/jnumed.116.178889.
- Roosenburg, S.; Laverman, P.; Van Delft, F.L.; Boerman, O.C. Radiolabeled CCK/gastrin peptides for imaging and therapy of CCK2 receptor-expressing tumors. Amino Acids 2011, 41, 1049–1058, doi:10.1007/s00726-010-0501-y.
- Kaeppeli, S.A.M.; Jodal, A.; Gotthardt, M.; Schibli, R.; Béhé, M. Exendin-4 Derivatives with an Albumin-Binding Moiety Show Decreased Renal Retention and Improved GLP-1 Receptor Targeting. Pharm. 2019, 16, 3760–3769, doi:10.1021/acs.molpharmaceut.9b00271.
- Li, M.; Liu, Y.; Xu, Y.; Yang, M. Preliminary evaluation of GLP-1R PET in the diagnosis and risk stratification of pheochromocytomas. Neoplasma 2020, 67, 27–36, doi:10.4149/neo_2019_190227N163.
- Nieberler, M.; Reuning, U.; Reichart, F.; Kessler, H. Exploring the Role of RGD-Recognizing Integrins in Cancer. Cancers 2017, 9, 116, doi:10.3390/cancers9090116.
- Ruan, Q.; Zhang, X.; Gan, Q.; Fang, S.; Zhang, J. Preparation of two 99mTc(CO)3 labelled complexes with a 4-nitroimidazole isocyanide at different temperatures for molecular imaging of tumor hypoxia. Radioanal. Nucl. Chem. 2020, 323, 851–859, doi:10.1007/s10967-019-07005-7.
- Ruan, Q.; Zhang, X.; Gan, Q.; Fang, S.; Zhang, J. Synthesis and evaluation of [99mTcN]2+ core and [99mTcO]3+ core labeled complexes with 4-nitroimidazole xanthate derivative for tumor hypoxia imaging. Med. Chem. Lett. 2020, 30, 127582, doi:10.1016/j.bmcl.2020.127582.
- Luo, Z.; Zhu, H.; Lin, X.; Chu, T.; Yang, Z. Synthesis and radiolabeling of 64Cu-labeled 2-nitroimidazole derivative 64Cu-BMS2P2 for hypoxia imaging. Med. Chem. Lett. 2016, 26, 1397–1400, doi:10.1016/j.bmcl.2016.01.077.
- Seelam, S.R.; Lee, J.Y.; Lee, Y.S.; Jeong, J.M. Development of 68Ga-labeled multivalent nitroimidazole derivatives for hypoxia imaging. Med. Chem. 2015, 23, 7743–7750, doi:10.1016/j.bmc.2015.11.024.
- Iikuni, S.; Ono, M.; Watanabe, H.; Saji, H. Cancer radiotheranostics targeting carbonic anhydrase-IX with 111In- and 90Y-labeled ureidosulfonamide scaffold for SPECT imaging and radionuclide-based therapy. Theranostics 2018, 8, 2992–3006, doi:10.7150/thno.20982.
- Nakai, M.; Pan, J.; Lin, K.S.; Storr, T. Evaluation of 99mTc-sulfonamide and sulfocoumarin derivatives for imaging carbonic anhydrase IX expression. Inorg. Biochem. 2018, 185, 63–70, doi:10.1016/j.jinorgbio.2018.04.009.
- Iikuni, S.; Tanimura, K.; Watanabe, H.; Shimizu, Y.; Saji, H.; Ono, M. Development of the 99mTc-Hydroxamamide Complex as a Probe Targeting Carbonic Anhydrase IX. Pharm. 2019, 16, 1489–1497, doi:10.1021/acs.molpharmaceut.8b01120.
- Lau, J.; Lin, K.S.; Bénard, F. Past, Present, and Future: Development of Theranostic Agents Targeting Carbonic Anhydrase IX. Theranostics 2017, 7, 4322–4339, doi:10.7150/thno.21848.
- Van Dijk, L.K.; Boerman, O.C.; Kaanders, J.H.A.M.; Bussink, J. Epidermal growth factor receptor imaging in human head and neck cancer xenografts. Acta Oncol. 2015, 54, 1263–1267, doi:10.3109/0284186X.2015.1063778.
- Obacz, J.; Pastorekova, S.; Vojtesek, B.; Hrstka, R. Cross-talk between HIF and p53 as mediators of molecular responses to physiological and genotoxic stresses. Cancer 2013, 12, 93, doi:10.1186/1476-4598-12-93.
- Mikulová, M.B.; Kružlicová, D.; Pecher, D.; Supuran, C.T.; Mikuš, P. Synthetic strategies and computational inhibition activity study for triazinyl-substituted benzenesulfonamide conjugates with polar and hydrophobic amino acids as inhibitors of carbonic anhydrases. J. Mol. Sci. 2020, 21, 3661, doi:10.3390/ijms21103661.
- Mhawech-Fauceglia, P.; Zhang, S.; Terracciano, L.; Penetrante, R. Prostate-specific membrane antigen (PSMA) protein expression in normal and neoplastic tissues and its sensitivity and specificity in prostate adenocarcinoma: An immunohistochemical study using mutiple tumour tissue microarray technique. Histopathology 2007, 50, 472–483, doi:10.1111/j.1365-2559.2007.02635.x.
- Wernicke, A.G.; Varma, S.; Greenwood, E.A.; Shin, S.J. Prostate-specific membrane antigen expression in tumor-associated vasculature of breast cancers. APMIS 2014, 122, 482–489, doi:10.1111/apm.12195.
- Haffner, M.C.; Kronberger, I.E.; Ross, J.S.; Bander, N.H. Prostate-specific membrane antigen expression in the neovasculature of gastric and colorectal cancers. Pathol. 2009, 40, 1754–1761, doi:10.1016/j.humpath.2009.06.003.
- Schmidt, L.H.; Heitkötter, B.; Schulze, A.B.; Huss, S. Prostate specific membrane antigen (PSMA) expression in non-small cell lung cancer. PLoS ONE 2017, 12, e0186280, doi:10.1371/journal.pone.0186280.
- Spatz, S.; Tolkach, Y.; Jung, K.; Kristiansen, G. Comprehensive evaluation of prostate specific membrane antigen expression in the vasculature of renal tumors: Implications for imaging studies and prognostic role. J. Urol. 2018, 199, 370–377, doi:10.1016/j.juro.2017.08.079.
- Nomura, N.; Pastorino, S.; Jiang, P.; Kesari, S. Prostate specific membrane antigen (PSMA) expression in primary gliomas and breast cancer brain metastases. Cancer Cell Int. 2014, 14, 26, doi:10.1186/1475-2867-14-26.
- Tolkach, Y.; Goltz, D.; Kremer, A.; Kristiansen, G. Prostate-specific membrane antigen expression in hepatocellular carcinoma: Potential use for prognosis and diagnostic imaging. Oncotarget 2019, 10, 4149–4160, doi:10.18632/oncotarget.27024.
- Busek, P.; Vanickova, Z.; Hrabal, P.; Sedo, A. Increased tissue and circulating levels of dipeptidyl peptidase-IV enzymatic activity in patients with pancreatic ductal adenocarcinoma. Pancreatology 2016, 16, 829–838, doi:10.1016/j.pan.2016.06.001.
- Shi, M.; Yu, D.H.; Chen, Y.; Zhu, M.H. Expression of fibroblast activation protein in human pancreatic adenocarcinoma and its clinicopathological significance. World J. Gastroenterol. 2012, 18, 840–846, doi:10.3748/wjg.v18.i8.840.
- Zou, B.; Liu, X.; Zhang, B.; Li, J. The Expression of FAP in Hepatocellular Carcinoma Cells is Induced by Hypoxia and Correlates with Poor Clinical Outcomes. Cancer 2018, 9, 3278–3286, doi:10.7150/jca.25775.
- Huang, Y.; Simms, A.E.; Mazur, A.; Kelly, T. Fibroblast activation protein-α promotes tumor growth and invasion of breast cancer cells through non-enzymatic functions. Exp. Metastasis 2011, 28, 567–579, doi:10.1007/s10585-011-9392-x.
- Wikberg, M.L.; Edin, S.; Lundberg, I.V.; Palmqvist, R. High intratumoral expression of fibroblast activation protein (FAP) in colon cancer is associated with poorer patient prognosis. Tumor Biol. 2013, 34, 1013–1020, doi:10.1007/s13277-012-0638-2.
- Mhawech-Fauceglia, P.; Yan, L.; Sharifian, M.; Lawrenson, K. Stromal Expression of Fibroblast Activation Protein Alpha (FAP) Predicts Platinum Resistance and Shorter Recurrence in patients with Epithelial Ovarian Cancer. Cancer Microenviron. 2015, 8, 23–31, doi:10.1007/s12307-014-0153-7.
- Yadav, D.; Ballal, S.; Yadav, M.P.; Tripathi, M.; Roesch, F.; Bal, C. Evaluation of [68Ga]Ga-DATA-TOC for imaging of neuroendocrine tumours: Comparison with [68Ga]Ga-DOTA-NOC PET/CT. J. Nucl. Med. Mol. Imaging 2020, 47, 860–869, doi:10.1007/s00259-019-04611-1.
- Touijer, K.A.; Michaud, L.; Vargas Alvarez, H.A.; Weber, W.A. Prospective Study of the Radiolabeled GRPR Antagonist BAY86-7548 for Positron Emission Tomography/Computed Tomography Imaging of Newly Diagnosed Prostate Cancer. Urol. Oncol. 2019, 2, 166–173, doi:10.1016/j.euo.2018.08.011.
- Hubalewska-Dydejczyk, A.; Mikolajczak, R.; Decristoforo, C. Radiolabelled CCK-2/Gastrin Receptor Analogue for Personalized Theranostic Strategy in Advanced MTC. 2020. Identifier: NCT03246659. Available online: https://clinicaltrials.gov/ (accessed on 30 Jan 2021).
- Vatsa, R.; Madaan, S.; Chakraborty, S.; Shukla, J. Clinical evaluation of kit based Tc-99m-HYNIC-RGD2 for imaging angiogenesis in breast carcinoma patients. Med. Commun. 2020, 41, 1250–1256, doi:10.1097/MNM.0000000000001282.
- Kulkarni, M.; Hughes, S.; Mallia, A.; Cook, G.J.R. The management impact of 68gallium-tris(hydroxypyridinone) prostate-specific membrane antigen (68Ga-THP-PSMA) PET-CT imaging for high-risk and biochemically recurrent prostate cancer. J. Nucl. Med. Mol. Imaging 2020, 47, 674–686, doi:10.1007/s00259-019-04643-7.
- Loktev, A.; Lindner, T.; Mier, W.; Haberkorn, U. A Tumor-Imaging Method Targeting Cancer-Associated Fibroblasts. Nucl. Med. 2018, 59, 1423–1429, doi:10.2967/jnumed.118.210435.
- Giesel, F.L.; Kratochwil, C.; Lindner, T.; Haberkorn, U. 68Ga-FAPI PET/CT: Biodistribution and Preliminary Dosimetry Estimate of 2 DOTA-Containing FAP-Targeting Agents in Patients with Various Cancers. Nucl. Med. 2019, 60, 386–392, doi:10.2967/jnumed.118.215913.
- Syed, M.; Flechsig, P.; Liermann, J.; Adeberg, S. Fibroblast activation protein inhibitor (FAPI) PET for diagnostics and advanced targeted radiotherapy in head and neck cancers. J. Nucl. Med. Mol. Imaging 2020, 47, 2836–2845, doi:10.1007/s00259-020-04859-y.
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Giesel, F.L. 68Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. Nucl. Med. 2019, 60, 801–805, doi:10.2967/jnumed.119.227967.
- Chen, H.; Zhao, L.; Ruan, D.; Wu, H. Usefulness of [68Ga]Ga-DOTA-FAPI-04 PET/CT in patients presenting with inconclusive [18F]FDG PET/CT findings. J. Nucl. Med. Mol. Imaging 2020, 48, 73–86, doi:10.1007/s00259-020-04940-6.
- Chen, H.; Pang, Y.; Wu, J.; Wu, H. Comparison of [68Ga]Ga-DOTA-FAPI-04 and [18F]FDG PET/CT for the diagnosis of primary and metastatic lesions in patients with various types of cancer. J. Nucl. Med. Mol. Imaging 2020, 47, 1820–1832, doi:10.1007/s00259-020-04769-z.
[1][2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][71][72][73][74][75][76][77][78][79][80][81][82][83][84][85][86][87][88]
References
- Okarvi, S. Peptide-based radiopharmaceuticals and cytotoxic conjugates. Cancer Treat. Rev. 2008, 34, 13–26, doi:10.1016/j.ctrv.2007.07.017.
- Brechbiel, M.W. Bifunctional Chelates for Metal Nuclides. J. Nucl. Med. Mol. Imaging 2008, 52, 166–173.
- Bartholoma, M. Recent Developments in the Design of Bifunctional Chelators for Metal-based Radiopharmaceuticals Used in Positron Emission Tomography. Chim. Acta 2012, 389, 36–51, doi:10.1016/j.ica.2012.01.061.
- Wängler, B.; Schirrmacher, R.; Bartenstein, P.; Wängler, C. Chelating Agents and their Use in Radiopharmaceutical Sciences. Mini Rev. Med. Chem. 2011, 11, 968–983, doi:10.2174/138955711797068445.
- Abrams, M.J.; Juweid, M.; Strauss, H.W.; Fischman, A.J. Technetium-99m-human polyclonal IgG radiolabeled via the hydrazino nicotinamide derivative for imaging focal sites of infection in rats. Nucl. Med. 1990, 31, 2022–2028.
- Dearling, J.L.J.; Blower, P.J. Redox-active metal complexes for imaging hypoxic tissues: Structure-activity relationships in copper(II) bis(thiosemicarbazone) complexes. Commun. 1998, 22, 2531–2532, doi:10.1039/A805957H.
- Blower, P.J.; Castle, T.C.; Cowley, A.R.; Went, M.J. Structural trends in copper(II) bis(thiosemicarbazone) radiopharmaceuticals. Dalton Trans. 2003, 23, 4416–4425, doi:10.1039/B307499D.
- Boswell, C.A.; Regino, C.A.S.; Baidoo, K.E.; Brechbiel, M.W. Synthesis of a cross-bridged cyclam derivative for peptide conjugation and 64Cu radiolabeling. Chem. 2008, 19, 1476–1484, doi:10.1021/bc800039e.
- Sun, X.; Wuest, M.; Weisman, G.R.; Anderson, C.J. Radiolabeling and in vivo behavior of copper-64-labeled cross-bridged cyclam ligands. Med. Chem. 2002, 45, 469–477, doi:10.1021/jm0103817.
- Tan, K.V.; Pellegrini, P.A.; Skelton, B.W.; Barnard, P.J. Triamidetriamine bearing macrobicyclic and macrotricyclic ligands: Potential applications in the development of copper-64 radiopharmaceuticals. Chem. 2014, 53, 468–477, doi:10.1021/ic4024508.
- Cai, H.; Fissekis, J.; Conti, P.S. Synthesis of a novel bifunctional chelator AmBaSar based on sarcophagine for peptide conjugation and 64Cu radiolabelling. Dalton Trans. 2009, 5395–5400, doi:10.1039/B902210D.
- Tsionou, M.I.; Knapp, C.E.; Foley, C.A; Ma, M.T. Comparison of macrocyclic and acyclic chelators for gallium-68 radiolabelling. RSC Adv. 2017, 7, 49586–49599, doi:10.1039/C7RA09076E.
- Fani, M.; Mäcke, H.R. Radiopharmaceutical development of radiolabelled peptides. J. Nucl. Med. Mol. Imaging 2012, 39, 11–30, doi:10.1007/s00259-011-2001-z.
- Liu, S. Bifunctional coupling agents for radiolabeling of biomolecules and target-specific delivery of metallic radionuclides. Drug Deliv. Rev. 2008, 60, 1347–1370, doi:10.1016/j.addr.2008.04.006.
- Heppeler, A.; Froidevaux, S.; Eberle, A.N.; Mäcke, H.R. Receptor Targeting for Tumor Localisation and Therapy with Radiopeptides. Med. Chem. 2000, 7, 971–994, doi:10.2174/0929867003374516.
- Dale, A.V.; An, G.I.; Pandya, D.N.; Yoo, J. Synthesis and Evaluation of New Generation Cross-Bridged Bifunctional Chelator for 64Cu Radiotracers. Chem. 2015, 54, 8177–8186, doi:10.1021/acs.inorgchem.5b01386.
- Deri, M.A.; Zeglis, B.M.; Francesconi, L.C.; Lewis, J.S. PET Imaging with 89Zr: From Radiochemistry to the Clinic. Med. Biol. 2013, 40, 3–14, doi:10.1016/j.nucmedbio.2012.08.004.
- Nwe, K.; Brechbiel, M.W. Growing Applications of “Click Chemistry” for Bioconjugation in Contemporary Biomedical Research. Cancer Biother. Radiopharm. 2009, 24, 289–302, doi:10.1089/cbr.2008.0626.
- Mindt, T.; Struthers, H.; Brans, L.; Schibli, R. “Click to Chelate”: Synthesis and Installation of Metal Chelates into Biomolecules in a Single Step. Am. Chem. Soc. 2006, 128, 15096–15097, doi:10.1021/ja066779f.
- Struthers, H.; Spingler, B.; Mindt, T.L.; Schibli, R. “Click-to-Chelate”: Design and Incorporation of Triazole-Containing Metal-Chelating Systems into Biomolecules of Diagnostic and Therapeutic Interest. Eur. J. 2008, 14, 6173–6183, doi:10.1002/chem.200702024.
- Meyer, J.P.; Adumeau, P.; Lewis, J.S.; Zeglis, B.M. Click Chemistry and Radiochemistry: The First 10 Years. Chem. 2016, 27, 2791–2807, doi:10.1021/acs.bioconjchem.6b00561.
- Mushtaq, S.; Yun, S.J.; Jeon, J. Recent Advances in Bioorthogonal Click Chemistry for Efficient Synthesis of Radiotracers and Radiopharmaceuticals. Molecules 2019, 24, 3567, doi:10.3390/molecules24193567.
- Svatunek, D.; Houszka, N.; Hamlin, T.A.; Bickelhaupt, F.M.; Mikula, H. Chemoselectivity of Tertiary Azides in Strain-Promoted Alkyne-Azide Cycloadditions. Eur. J. 2019, 25, 754–758, doi:10.1002/chem.201805215.
- Makarem, A.; Sarvestani, M.K.; Klika, K.D.; Kopka, K. A Multifunctional HBED-Type Chelator with Dual Conjugation Capabilities for Radiopharmaceutical Development. Synlett 2019, 30, doi:10.1055/s-0039-1690194.
- Baranyai, Z.; Reich, D.; Vagner, A.; Notni, J. A shortcut to high-affinity Ga-68 and Cu-64 radiopharmaceuticals: One-pot click chemistry trimerisation on the TRAP platform. Dalton Trans. 2015, 44, 11137–11146, doi:10.1039/C5DT00576K.
- Radford, L.L.; Papagiannopoulou, D.; Gallazzi, F.; Hennkens, H.M. Synthesis and evaluation of Re/99mTc(I) complexes bearing a somatostatin receptor-targeting antagonist and labeled via a novel [N,S,O] clickable bifunctional chelating agent. Med. Chem. 2019, 27, 492–501, doi:10.1016/j.bmc.2018.12.028.
- Summer, D.; Mayr, S.; Petrik, M.; Decristoforo, C. Pretargeted Imaging with Gallium-68—Improving the Binding Capability by Increasing the Number of Tetrazine Motifs. Pharmaceuticals 2018, 11, 102, doi:10.3390/ph11040102.
- Chen, K.T.; Nguyen, K.; Ieritano, C.; Gao, F.; Seimbille, Y. A Flexible Synthesis of 68Ga-Labeled Carbonic Anhydrase IX (CAIX)-Targeted Molecules via CBT/1,2-Aminothiol Click Reaction. Molecules 2019, 24, 23, doi:10.3390/molecules24010023.
- Wall, M.; Shäffer, T.M.; Harmsen, S.; Kircher, M. Chelator-Free Radiolabeling of SERRS Nanoparticles for Whole-Body PET and Intraoperative Raman Imaging. Theranostics 2017, 3068–3077, doi:10.7150/thno.18019.
- Varani, M.; Galli, F.; Auletta, S.; Signore, A. Radiolabelled nanoparticles for cancer diagnosis. Transl. Imaging 2018, 6, 271–292, doi:10.1007/s40336-018-0283-x.
- Morales-Avila, E.; Ferro-Flores, G.; Gomez-Olivan, L. Multimeric system of 99mTc-labeled gold nanoparticles conjugated to c[RGDfK(C)] for molecular imaging of tumor α(v)β(3) expression. Chem. 2011, 22, 913–922, doi:10.1021/bc100551s.
- Peiris, P.M.; Deb, P.; Doolittle, E.; Karathanasis, E. Vascular targeting of a gold nanoparticle to breast cancer metastasis. Pharm. Sci. 2015, 104, 2600–2610, doi:10.1002/jps.24518.
- Mendoza-Sanchez, A.N.; Ferro-Flores, G.; Camacho-Lopez, M.A. Lys3-bombesin conjugated to 99mTc-labelled gold nanoparticles for in vivo gastrin releasing peptide-receptor imaging. Biomed. Nanotechnol. 2010, 6, 375–384, doi:10.1166/jbn.2010.1132.
- Silva, F.; Gano, L.; Campello, M.P.C.; Kannan, R. In vitro/in vivo “peeling” of multilayered aminocarboxylate gold nanoparticles evidenced by a kinetically stable 99mTc-label. Dalton Trans. 2017, 46, 14572–14583, doi:10.1039/C7DT00864C.
- Orocio-Rodriguez, E.; Ferro-Flores, G.; Sanchez-Garcia, F.M. Two novel nanosized radiolabeled analogues of somatostatin for neuroendocrine tumor imaging. Nanosci. Nanotechnol. 2015, 15, 4159–4169, doi:10.1166/jnn.2015.9620.
- Xiao, Y.; Hong, H.; Matson, V.Z.; Gong, S. Gold Nanorods Conjugated with Doxorubicin and cRGD for Combined Anticancer Drug Delivery and PET Imaging. Theranostics 2012, 2, 757–768, doi:10.7150/thno.4756.
- Cai, H.; Xie, F.; Mulgaonkar, A.; Wu, C.; Ai, H. Bombesin functionalized 64Cu-copper sulfide nanoparticles for targeted imaging of orthotopic prostate cancer. Nanomedicine 2018, 13, 1695–1705, doi:10.2217/nnm-2018-0062.
- Chilug, L.E.; Leonte, R.A.; Patrascu, M.E.B.; Niculae, D. In vitro binding kinetics study of gold nanoparticles functionalized with 68Ga-DOTA conjugated peptides. Radioanal. Nucl. Chem. 2017, 311, 1485–1493, doi:10.1007/s10967-016-5075-z.
- Lahooti, A.; Shanehsazzadeh, S.; Laurent, S. Preliminary studies of 68Ga-NODA-USPION-BBN as a dual-modality contrast agent for use in positron emission tomography/magnetic resonance imaging. Nanotechnology 2020, 31, 015102, doi:10.1088/1361-6528/ab4446.
- Hajiramezanali, M.; Atyabi, F.; Mosayebnia, M.; Beiki, D. 68Ga-radiolabeled bombesin-conjugated to trimethyl chitosan-coated superparamagnetic nanoparticles for molecular imaging: Preparation, characterization and biological evaluation. J. Nanomed. 2019, 14, 2591–2605, doi:10.2147/IJN.S195223.
- Nicolas, G.P.; Schreiter, N.; Kaul, F.; Wild, D. Sensitivity comparison of 68Ga-OPS202 and 68Ga-DOTATOC PET/CT in patients with gastroenteropancreatic neuroendocrine tumors: A prospective phase II imaging study. Nucl. Med. 2018, 59, 915–921, doi:10.2967/jnumed.117.199760.
- Cuccurullo, V.; Prisco, M.R.; Di Stasio, G.D.; Mansi, L. Nuclear Medicine in Patients with NET: Radiolabeled Somatostatin Analogues and their Brothers. Radiopharm. 2017, 10, 74–84, doi:10.2174/1874471010666170323115136.
- Cakir, M.; Dworakowska, D.; Grossman, A. Somatostatin receptor biology in neuroendocrine and pituitary tumours: Part 2—Clinical implications. Cell. Mol. Med. 2010, 14, 2585–2591, doi:10.1111/j.1582-4934.2010.01125_1.x.
- Orlando, C.; Raggi, C.C.; Bianchi, S.; Serio, M. Measurement of somatostatin receptor subtype 2 mRNA in breast cancer and corresponding normal tissue. Relat. Cancer 2004, 11, 323–332, doi:10.1677/erc.0.0110323.
- Lapa, C.; Hänscheid, H.; Wild, V.; Lückerath, K. Somatostatin receptor expression in small cell lung cancer as a prognostic marker and a target for peptide receptor radionuclide therapy. Oncotarget 2016, 7, 20033–20040, doi:10.18632/oncotarget.7706.
- Lum, S.S.; Fletcher, W.S.; O’Dorisio, M.S.; Caprara, M. Distribution and functional significance of somatostatin receptors in malignant melanoma. World J. Surg. 2001, 25, 407–412, doi:10.1007/s002680020102.
- Moreno, P.; Ramos-Alvarez, I.; Moody, T.W.; Jensen, R.T. Bombesin related peptides/receptors and their promising therapeutic roles in cancer imaging, targeting and treatment. Expert Opin. Ther. Targets 2016, 20, 1055–1073, doi:10.1517/14728222.2016.1164694.
- Baratto, L.; Jadvar, H.; Iagaru, A. Prostate Cancer Theranostics Targeting Gastrin-Releasing Peptide Receptors. Imaging Biol. 2017, 20, 501–509, doi:10.1007/s11307-017-1151-1.
- Nock, B.A.; Kaloudi, A.; Lymperis, E.; Maina, T.; Baum, R.P. Theranostic Perspectives in Prostate Cancer with the Gastrin-Releasing Peptide Receptor Antagonist NeoBOMB1: Preclinical and First Clinical Results. Nucl. Med. 2017, 58, 75–80, doi:10.2967/jnumed.116.178889.
- Roosenburg, S.; Laverman, P.; Van Delft, F.L.; Boerman, O.C. Radiolabeled CCK/gastrin peptides for imaging and therapy of CCK2 receptor-expressing tumors. Amino Acids 2011, 41, 1049–1058, doi:10.1007/s00726-010-0501-y.
- Kaeppeli, S.A.M.; Jodal, A.; Gotthardt, M.; Schibli, R.; Béhé, M. Exendin-4 Derivatives with an Albumin-Binding Moiety Show Decreased Renal Retention and Improved GLP-1 Receptor Targeting. Pharm. 2019, 16, 3760–3769, doi:10.1021/acs.molpharmaceut.9b00271.
- Li, M.; Liu, Y.; Xu, Y.; Yang, M. Preliminary evaluation of GLP-1R PET in the diagnosis and risk stratification of pheochromocytomas. Neoplasma 2020, 67, 27–36, doi:10.4149/neo_2019_190227N163.
- Nieberler, M.; Reuning, U.; Reichart, F.; Kessler, H. Exploring the Role of RGD-Recognizing Integrins in Cancer. Cancers 2017, 9, 116, doi:10.3390/cancers9090116.
- Ruan, Q.; Zhang, X.; Gan, Q.; Fang, S.; Zhang, J. Preparation of two 99mTc(CO)3 labelled complexes with a 4-nitroimidazole isocyanide at different temperatures for molecular imaging of tumor hypoxia. Radioanal. Nucl. Chem. 2020, 323, 851–859, doi:10.1007/s10967-019-07005-7.
- Ruan, Q.; Zhang, X.; Gan, Q.; Fang, S.; Zhang, J. Synthesis and evaluation of [99mTcN]2+ core and [99mTcO]3+ core labeled complexes with 4-nitroimidazole xanthate derivative for tumor hypoxia imaging. Med. Chem. Lett. 2020, 30, 127582, doi:10.1016/j.bmcl.2020.127582.
- Luo, Z.; Zhu, H.; Lin, X.; Chu, T.; Yang, Z. Synthesis and radiolabeling of 64Cu-labeled 2-nitroimidazole derivative 64Cu-BMS2P2 for hypoxia imaging. Med. Chem. Lett. 2016, 26, 1397–1400, doi:10.1016/j.bmcl.2016.01.077.
- Seelam, S.R.; Lee, J.Y.; Lee, Y.S.; Jeong, J.M. Development of 68Ga-labeled multivalent nitroimidazole derivatives for hypoxia imaging. Med. Chem. 2015, 23, 7743–7750, doi:10.1016/j.bmc.2015.11.024.
- Iikuni, S.; Ono, M.; Watanabe, H.; Saji, H. Cancer radiotheranostics targeting carbonic anhydrase-IX with 111In- and 90Y-labeled ureidosulfonamide scaffold for SPECT imaging and radionuclide-based therapy. Theranostics 2018, 8, 2992–3006, doi:10.7150/thno.20982.
- Nakai, M.; Pan, J.; Lin, K.S.; Storr, T. Evaluation of 99mTc-sulfonamide and sulfocoumarin derivatives for imaging carbonic anhydrase IX expression. Inorg. Biochem. 2018, 185, 63–70, doi:10.1016/j.jinorgbio.2018.04.009.
- Iikuni, S.; Tanimura, K.; Watanabe, H.; Shimizu, Y.; Saji, H.; Ono, M. Development of the 99mTc-Hydroxamamide Complex as a Probe Targeting Carbonic Anhydrase IX. Pharm. 2019, 16, 1489–1497, doi:10.1021/acs.molpharmaceut.8b01120.
- Lau, J.; Lin, K.S.; Bénard, F. Past, Present, and Future: Development of Theranostic Agents Targeting Carbonic Anhydrase IX. Theranostics 2017, 7, 4322–4339, doi:10.7150/thno.21848.
- Van Dijk, L.K.; Boerman, O.C.; Kaanders, J.H.A.M.; Bussink, J. Epidermal growth factor receptor imaging in human head and neck cancer xenografts. Acta Oncol. 2015, 54, 1263–1267, doi:10.3109/0284186X.2015.1063778.
- Obacz, J.; Pastorekova, S.; Vojtesek, B.; Hrstka, R. Cross-talk between HIF and p53 as mediators of molecular responses to physiological and genotoxic stresses. Cancer 2013, 12, 93, doi:10.1186/1476-4598-12-93.
- Mikulová, M.B.; Kružlicová, D.; Pecher, D.; Supuran, C.T.; Mikuš, P. Synthetic strategies and computational inhibition activity study for triazinyl-substituted benzenesulfonamide conjugates with polar and hydrophobic amino acids as inhibitors of carbonic anhydrases. J. Mol. Sci. 2020, 21, 3661, doi:10.3390/ijms21103661.
- Mhawech-Fauceglia, P.; Zhang, S.; Terracciano, L.; Penetrante, R. Prostate-specific membrane antigen (PSMA) protein expression in normal and neoplastic tissues and its sensitivity and specificity in prostate adenocarcinoma: An immunohistochemical study using mutiple tumour tissue microarray technique. Histopathology 2007, 50, 472–483, doi:10.1111/j.1365-2559.2007.02635.x.
- Wernicke, A.G.; Varma, S.; Greenwood, E.A.; Shin, S.J. Prostate-specific membrane antigen expression in tumor-associated vasculature of breast cancers. APMIS 2014, 122, 482–489, doi:10.1111/apm.12195.
- Haffner, M.C.; Kronberger, I.E.; Ross, J.S.; Bander, N.H. Prostate-specific membrane antigen expression in the neovasculature of gastric and colorectal cancers. Pathol. 2009, 40, 1754–1761, doi:10.1016/j.humpath.2009.06.003.
- Schmidt, L.H.; Heitkötter, B.; Schulze, A.B.; Huss, S. Prostate specific membrane antigen (PSMA) expression in non-small cell lung cancer. PLoS ONE 2017, 12, e0186280, doi:10.1371/journal.pone.0186280.
- Spatz, S.; Tolkach, Y.; Jung, K.; Kristiansen, G. Comprehensive evaluation of prostate specific membrane antigen expression in the vasculature of renal tumors: Implications for imaging studies and prognostic role. J. Urol. 2018, 199, 370–377, doi:10.1016/j.juro.2017.08.079.
- Nomura, N.; Pastorino, S.; Jiang, P.; Kesari, S. Prostate specific membrane antigen (PSMA) expression in primary gliomas and breast cancer brain metastases. Cancer Cell Int. 2014, 14, 26, doi:10.1186/1475-2867-14-26.
- Tolkach, Y.; Goltz, D.; Kremer, A.; Kristiansen, G. Prostate-specific membrane antigen expression in hepatocellular carcinoma: Potential use for prognosis and diagnostic imaging. Oncotarget 2019, 10, 4149–4160, doi:10.18632/oncotarget.27024.
- Busek, P.; Vanickova, Z.; Hrabal, P.; Sedo, A. Increased tissue and circulating levels of dipeptidyl peptidase-IV enzymatic activity in patients with pancreatic ductal adenocarcinoma. Pancreatology 2016, 16, 829–838, doi:10.1016/j.pan.2016.06.001.
- Shi, M.; Yu, D.H.; Chen, Y.; Zhu, M.H. Expression of fibroblast activation protein in human pancreatic adenocarcinoma and its clinicopathological significance. World J. Gastroenterol. 2012, 18, 840–846, doi:10.3748/wjg.v18.i8.840.
- Zou, B.; Liu, X.; Zhang, B.; Li, J. The Expression of FAP in Hepatocellular Carcinoma Cells is Induced by Hypoxia and Correlates with Poor Clinical Outcomes. Cancer 2018, 9, 3278–3286, doi:10.7150/jca.25775.
- Huang, Y.; Simms, A.E.; Mazur, A.; Kelly, T. Fibroblast activation protein-α promotes tumor growth and invasion of breast cancer cells through non-enzymatic functions. Exp. Metastasis 2011, 28, 567–579, doi:10.1007/s10585-011-9392-x.
- Wikberg, M.L.; Edin, S.; Lundberg, I.V.; Palmqvist, R. High intratumoral expression of fibroblast activation protein (FAP) in colon cancer is associated with poorer patient prognosis. Tumor Biol. 2013, 34, 1013–1020, doi:10.1007/s13277-012-0638-2.
- Mhawech-Fauceglia, P.; Yan, L.; Sharifian, M.; Lawrenson, K. Stromal Expression of Fibroblast Activation Protein Alpha (FAP) Predicts Platinum Resistance and Shorter Recurrence in patients with Epithelial Ovarian Cancer. Cancer Microenviron. 2015, 8, 23–31, doi:10.1007/s12307-014-0153-7.
- Yadav, D.; Ballal, S.; Yadav, M.P.; Tripathi, M.; Roesch, F.; Bal, C. Evaluation of [68Ga]Ga-DATA-TOC for imaging of neuroendocrine tumours: Comparison with [68Ga]Ga-DOTA-NOC PET/CT. J. Nucl. Med. Mol. Imaging 2020, 47, 860–869, doi:10.1007/s00259-019-04611-1.
- Touijer, K.A.; Michaud, L.; Vargas Alvarez, H.A.; Weber, W.A. Prospective Study of the Radiolabeled GRPR Antagonist BAY86-7548 for Positron Emission Tomography/Computed Tomography Imaging of Newly Diagnosed Prostate Cancer. Urol. Oncol. 2019, 2, 166–173, doi:10.1016/j.euo.2018.08.011.
- Hubalewska-Dydejczyk, A.; Mikolajczak, R.; Decristoforo, C. Radiolabelled CCK-2/Gastrin Receptor Analogue for Personalized Theranostic Strategy in Advanced MTC. 2020. Identifier: NCT03246659. Available online: https://clinicaltrials.gov/ (accessed on 30 Jan 2021).
- Vatsa, R.; Madaan, S.; Chakraborty, S.; Shukla, J. Clinical evaluation of kit based Tc-99m-HYNIC-RGD2 for imaging angiogenesis in breast carcinoma patients. Med. Commun. 2020, 41, 1250–1256, doi:10.1097/MNM.0000000000001282.
- Kulkarni, M.; Hughes, S.; Mallia, A.; Cook, G.J.R. The management impact of 68gallium-tris(hydroxypyridinone) prostate-specific membrane antigen (68Ga-THP-PSMA) PET-CT imaging for high-risk and biochemically recurrent prostate cancer. J. Nucl. Med. Mol. Imaging 2020, 47, 674–686, doi:10.1007/s00259-019-04643-7.
- Loktev, A.; Lindner, T.; Mier, W.; Haberkorn, U. A Tumor-Imaging Method Targeting Cancer-Associated Fibroblasts. Nucl. Med. 2018, 59, 1423–1429, doi:10.2967/jnumed.118.210435.
- Giesel, F.L.; Kratochwil, C.; Lindner, T.; Haberkorn, U. 68Ga-FAPI PET/CT: Biodistribution and Preliminary Dosimetry Estimate of 2 DOTA-Containing FAP-Targeting Agents in Patients with Various Cancers. Nucl. Med. 2019, 60, 386–392, doi:10.2967/jnumed.118.215913.
- Syed, M.; Flechsig, P.; Liermann, J.; Adeberg, S. Fibroblast activation protein inhibitor (FAPI) PET for diagnostics and advanced targeted radiotherapy in head and neck cancers. J. Nucl. Med. Mol. Imaging 2020, 47, 2836–2845, doi:10.1007/s00259-020-04859-y.
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Giesel, F.L. 68Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. Nucl. Med. 2019, 60, 801–805, doi:10.2967/jnumed.119.227967.
- Chen, H.; Zhao, L.; Ruan, D.; Wu, H. Usefulness of [68Ga]Ga-DOTA-FAPI-04 PET/CT in patients presenting with inconclusive [18F]FDG PET/CT findings. J. Nucl. Med. Mol. Imaging 2020, 48, 73–86, doi:10.1007/s00259-020-04940-6.
- Chen, H.; Pang, Y.; Wu, J.; Wu, H. Comparison of [68Ga]Ga-DOTA-FAPI-04 and [18F]FDG PET/CT for the diagnosis of primary and metastatic lesions in patients with various types of cancer. J. Nucl. Med. Mol. Imaging 2020, 47, 1820–1832, doi:10.1007/s00259-020-04769-z.