For nearly a decade, researchers in the field of pediatric oncology have been using zebrafish as a model for understanding the contributions of genetic alternations to the pathogenesis of neuroblastoma (NB), and exploring the molecular and cellular mechanisms that underlie neuroblastoma initiation and metastasis.
- neuroblastoma
- zebrafish
- animal model
1. Introduction
Over the past ten years, zebrafish have become an increasingly popular tool for scientists conducting biomedical studies and other research. The species’ high fecundity rate, low cost of maintenance, and the ease of observation and genetic manipulation all contribute to its increasing use as an alternative and valuable vertebrate model system to study human disease. The expanding community of researchers using zebrafish has brought advanced technologies to the model, as well as a rapidly expanding inventory of transgenic and mutant lines that can be applied to different research niches. Cancer research using the zebrafish model can be traced back to 1965, when Dr. Mearle Stantion performed pioneered work to induce hepatic neoplasia in zebrafish with Diethylnitrosamine [1]. In 2003, the first zebrafish genetic cancer model was reported by Drs. David Langenau and Thomas Look, in which the MYC oncogene was overexpressed under control of the rag2 promoter, resulting in the development of T cell leukemia in the transgenic animal [2]. Since then many more zebrafish cancer models have been developed to understand the pathogenesis of leukemia, melanoma, rhabdomyosarcoma, hepatocellular carcinoma and many other tumor types [3][4][5][6]. In particular, the zebrafish model has also shown exceptional promise in dissecting the contributions of genetic alterations that were identified from integrative genomic analyses of neuroblastoma (NB) to the pathogenesis of this devastating pediatric cancer.
NB is the most common extracranial solid tumor in children and accounts for ~10% of all childhood cancer-related deaths [7]. It is derived from transformed neural crest progenitor cells in the developing peripheral sympathetic nervous system (PSNS) [8][9]. High-risk patients with amplified MYCN and over 18 months of age are often presented with widespread metastasis at diagnosis. Over the past few years, the five-year event-free survival rate for children with high-risk disease remains lower than 50% [10][11]. Very recently, a Phase III trial of immunotherapy, consisting of Dinutuximab, granulocyte macrophage-colony stimulating factor (GM-CSF) and interleukin-2 (IL2), showed significantly increased five-year overall survival rate of patients with high-risk NB to ~70% [12][13]. This immunotherapy has been approved by FDA for the treatment of patients with high-risk NB who achieve at least a partial response to prior first-line multiagent, multimodality therapy [12]. Although the improved outcomes are observed with the inclusion of Dinutuximab as part of treatment regimens for newly diagnosed NB, the prognosis for the relapsed disease remains poor (<10% progression-free survival) [14][15]. Therefore, better understanding of the pathogenesis of this disease and developing novel and more effective therapies are needed.
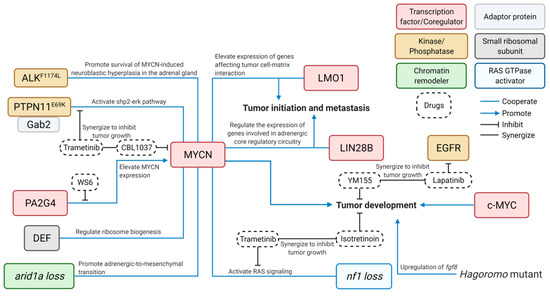
As an important member of the MYC proto-oncogene family identified from NB patients [16], MYCN amplification accounts for ~25% of NB cases and is associated with poor disease outcome [17][18][19]. MYCN is a bHLH transcription factor and is homologous to c-MYC structurally and functionally. It can promote neoplastic transformation of cultured mammalian cells and rat embryo fibroblasts [20][21]. In 1997, Dr. William Weiss developed the first animal model of NB by overexpressing MYCN under control of Tyrosine Hydroxylase (TH) in transgenic mice, which is by far still the most popular model for NB research [22]. Following Dr. Weiss’s effort, several genetically modified mouse (GEMM) lines with direct, conditional, inducible overexpression, knock-in or knockout of NB-relevant genes, including mutationally activated ALK (Anaplastic Lymphoma Receptor Tyrosine Kinase) [23][24][25], LIN28B (Lin-28 Homolog B) [26][27], SV40 large T antigen (Simian Vacuolating Virus 40 TAg) [28][29][30] and others [31][32] were subsequently developed. These models demonstrated a sufficient induction of NB in mice, which resemble the features of human NBs [24].
Although the mouse model provides valuable molecular insights on NB pathogenesis and opened the door for NB research, it has some disadvantages when compared to the zebrafish model. Neuroblastomas are different from adult tumors, in that they arise early in development; identifying the early onset of tumorigenesis in mice without euthanizing the animals is difficult and creates challenges in dissecting the molecular and cellular mechanisms underlying early onset tumor initiation. Zebrafish, by contrast, are translucent and develop from externally fertilized eggs, which allows for early detection of tumor onset in live animals. The zebrafish model is also more practical than GEMM, less expensive, and does not require sacrificing these animals to track tumor initiation and visualization of tumor growth. Therefore, the zebrafish model can serve as an alternative for the commonly used mouse model to conduct genetic research.
In 2012, the first zebrafish model of NB was generated and published by Zhu et al. [33]. Two oncogenes, MYCN and mutationally activated ALK (the most commonly mutated genes in primary neuroblastoma [34][35][36][37] and an attractive candidate for targeted therapy [38][39]), were expressed under control of the dopamine-beta-hydroxylase (dβh) promoter [33]. Following this initial effort on modeling NB in zebrafish, many new transgenic fish lines were developed, uncovering additional novel genetic alterations that cooperate with MYCN or c-MYC during NB pathogenesis. The evolution of zebrafish NB models has revealed the complexity of this disease at the molecular level and demonstrates the robustness of the model system in deepening our understanding of the molecular and cellular basis underlying NB pathogenesis. An overview of the NB zebrafish disease model workflow is illustrated in Figure 1.
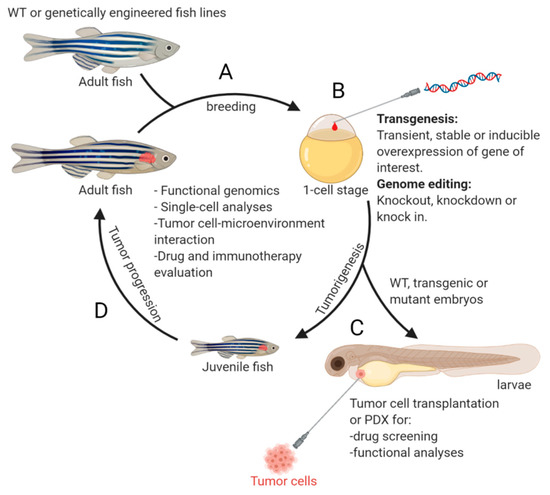
Of course, the advances in understanding NB have not been achieved without obstacles and challenges, some of which appear daunting. In this paper, we aim to:
-
Compare and contrast the zebrafish model with other popular lab animals used as disease models in order to help cancer and other biomedical researchers determine appropriate models for experimental applications (Table 1); and
| Zebrafish | Mouse | Fly | Worm | ||||||
|---|---|---|---|---|---|---|---|---|---|
| New Models Developed | Drugs Tested in the Zebrafish Models | Drugs applied in NB Treatment, Clinical Trials or other Animal Models | |||||||
| Transparency | Fully transparent at embryonic stage and remain translucency through adulthood. PTU can be used to inhibit pigmentation during early embryonic development. Mutant fish line without pigments are available. |
Not transparent | Transparent in larva stage and some parts of the adults | Transparent No pigmentation |
|||||
| Amsterdam, A. et al., 2009 [40] | Retroviral-mediated mutagenesis | Hagoromo Mutants | N/A | N/A | |||||
| Offspring size per mating | Up to 100 | ~3–12 | Up to 500 | Hermaphrodites, varies | |||||
| Zhu, S. et al., 2012 [33] | I-SceI meganuclease mediated transgenesis | Tg(dβh:EGFP-MYCN) and Tg(dβh:EGFP; dβh:ALKF1174L) transgenic fish lines | N/A | N/A | Genetic similarity (humans genome as reference) |
71% | 85% | 50% | 52% |
| Pei, D. et al., 2013 [41] | Morpholino-mediated gene knockdown & transient overexpression of structure variants | Embryos with gain or loss of function of phox2b/PHOX2B | 13–cis retinoic acid (at 1~100 nM) treatment of embryos | Applied to patients with high-risk NB as maintenance therapy after consolidation therapy [42][43] | Immune System | Underdeveloped adaptive immune system in larvae | |||
| He, S. et al., 2016 [44] | I-SceI meganuclease mediated transgenesis | Intact | Does not possess acquired/adaptive immunity | Does not possess acquired/adaptive immunity | |||||
| Tg(dβh: GRD; dβh:mCherry | ) transgenic fish line | Isotretinoin (13-cis retinoic acid, at 1~2 µM) and Trametinib (MEK inhibitor, at 10~40 nM) treatment of juvenile fish | Trametinib is in clinical trials for the treatments of various types of cancers, including high-risk NB (see NCI clinical trial information). | Tumor visualization | Directly visualized in vivo by microscopy | Cannot be easily visualized inside the body | Directly visualized in vivo by microscopy | Directly visualized in vivo by microscopy | |
| Zhang, X. et al., 2017 [45] | I-SceI meganuclease mediated transgenesis | Tg(dβh:Gab2wt;dβh:EGFP) and Tg(dβh:ptpn11E69K-EGFP) transgenic fish lines | CBL0137 (FACT inhibitor, at 4 mM) and Trametinib (MEK inhibitor, at 2 μM) treatment of tumor-bearing fish | CBL0137 is in a clinical trial for the treatment of patients with advanced extremity melanoma or sarcoma with metastasis (see NCI clinical trial information). In TH-MYCN tumor-bearing mice, CBL0137 combined with panobinostat can ablate tumor completely (Oncology Times: December 20, 2018) |
Gene editing tools | ||||
| Morpholino | |||||||||
| Zhu, S. et al., 2017 [46] | I-SceI meganuclease mediated transgenesis | Tg(dβh:LMO1;dβh:mCherry) transgenic fish line | N/A | N/A | Established | Feasible but very limited | |||
| Radic-Sarikas, B. et al., 2017 [47 | Possible but not done yet | ] | Drug treatment | Possible but not done yet | |||||
| N/A | Lapatinib (EGFR inhibitor, at 2 µM) and YM155 (ABCB1 blocker, at 6.5 nM) treatment of tumor-bearing adult fish | Lapatinib is in clinical trials for the treatments of various types of cancers (see NCI clinical trial information). | Retroviral insertion mutagenesis screen | Feasible | Established | dβh:MYCNFeasible | ) transgenic fish linesFeasible | ||
| N/A | N/A | DNA co-injection (I-SceI) Transgenesis | Established, high efficiency | Hypothetical and not efficient | Hypothetical | ||||
| Zimmerman, M. W. et al., 2018 | Possible | ||||||||
| [49 | CRISPR/TALENs | Established | Established | Established | Established | ||||
| Tao, T. et al., 2017 [48] | I-SceI meganuclease mediated transgenesis | Tg(dβh:mCherry;dβh:DEF) and Tg(dβh:EGFP;] | I-SceI meganuclease mediated transgenesis | Tg(dβh:c-MYC; dβh:mCherry) transgenic fish line | N/A | N/A | |||
| Shen, J. et al., 2018 [50] | Injection of tumor cells into the yolk sac of zebrafish embryos | Zebrafish embryos xenografted with SK-N-BE(2)-C human NB cell line | Crizotinib (ALK/MET inhibitor, at 8 μM) and 20a (histone deacetylase inhibitor, at 100 μM) treatment of embryos transplanted with SK-N-BE(2)-C human NB cells. | Crizotinib is in clinical trials for the treatments of various types of cancers, including high-risk NB (see NCI clinical trial information). | Tumor transplantation/Xenograft application | Efficient | Moderate to difficult | N/A | N/A |
| Aveic, S. et al., 2018 [51] | Injection of tumor cells into the duct of Cuvier of zebrafish embryos | Tg(fli1:GFP) zebrafish embryos transplanted with NB3 and SH-SY5Y NB cell lines | TP-0903 (multi-kinase inhibitor) treatment of embryos transplanted with NB3 and SH-SY5Y NB cell lines | TP-0903 is in a clinical trial for the treatment of FLT3 mutated acute myeloid leukemia (see NCI clinical trial information). | Chimeric animal development | Mouse-zebrafish Chimeric | Human-mouse Chimeric | N/A | N/A |
| Seda, M. et al., 2019 [52] | Compound screen using Tg(sox10:gfp) transgenic larvae | N/A | Leflunomide was one of the top hits identified from a library of 640 compounds to regulate cartilage remodelling and NB cell viability. | Leflunomide is approved by FDA for the treatment of active rheumatoid arthritis. It is also in clinical trials for the treatments of various types of cancers (see NCI clinical trial information). | Syngeneic model | Yes | Yes | Yes | N/A |
| Drug screening | Established, high-throughput | Established, low-throughput | Established, high-throughput | Established, high-throughput |
| Publications | Approaches | |||
|---|---|---|---|---|
| Koach, J. et al., 2019 | ||||
| [ | ||||
| 53 | ||||
| ] | ||||
| Tol2 transposon- mediated transgenesis | ||||
| Tg(dβh:PA2G4) | ||||
| transgenic fish line | ||||
| WS6 (175.4 mg/kg, 5 μL) treatment of tumor-bearing fish | ||||
| WS6 can also suppress tumor growth in the | TH | - | MYCN | mouse model and mice xenografted with human NB cell lines [53]. |
| Tao, T. et al., 2020 [54] | I-SceI meganuclease mediated transgenesis | Tg(dβh:EGFP;dβh:LIN28B_WT) and Tg(dβh:EGFP;dβh:LIN28B_MU) transgenic fish lines | N/A | N/A |
| Shi, H. et al., 2020 [55] | CRISPR/Cas9-mediate gene knockout | arid1aa and arid1ab knockout fish lines | N/A | N/A |
| Dong, Z. et al., 2021 [56] | TALEN-mediate gene knockout | gas7 knockout fish line | N/A | N/A |
Since topics related to PSNS development in zebrafish and mammals and NB genetics have been previously covered in detail by ourselves and others [57][58], we will not be addressing these subjects in this review.
2. Advantages of Using Zebrafish as a Model for NB Research
2.1. Translucency of Juvenile and Adult Fish
2.1.1. Early Detection of Tumor Onset
TH-MYCN
[22]
TH-MYCN
2.1.2. Real-Time Monitoring of Tumor Progression and Metastasis
MYCN
LMO1
[46]
[61]
[62]
[46]
gas7
[56]
[63]
[64]
[65]
[69]
MYCN
LMO1
[46]
2.1.3. Efficient Evaluation of the Efficacy of Drug Treatment
dβh-MYCN
MYCN
[47]
MYCN
MYCN
PA2G4
[53]
MYCN
MYCN
GAB2
MYCN
GAB2
[45]
nf1
MYCN
[44]
2.2. Robustness in Genome Editing and Manipulation of Gene Expression
[72]
2.2.1. Retroviral-Mediated Mutagenesis
[73]
[74]
fgf8
[40]
2.2.2. I-SceI Meganuclease-Mediated Transgenesis
MYCN
ALKF1174L
[33]
TH-MYCN
TH
dβh
[33]
dβh-EGFP-MYCN
[33]
dβh-EGFP
dβh-mCherry
dβh
[33]
[33]
EGFP
[33]
mCherry
[46]
ALKwt
[33]
PTPN11
[45]
GAB2
[45]
LIN28B WT
[54]
LIN28B_MU
[54]
LMO1
[46]
DEF
[48]
MYCN
dβh
[48]
dβh-EGFP-MYCN
dβh-MYCN
dβh-EGFP
TgMYCN_TT
TgMYCN_TT
MYCN
MYCN
[77]
MYCN
TgMYCN_TT
[48]
TgMYCN_TT
MYCN
[46]
c-MYC
MYCN
[49]
2.2.3. Genome Editing with Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) and Transcription Activator-Like Effector Nucleases (TALENs)
[80]
[81]
arid1aa
arid1ab
MYCN
ARID1A
arid1aa
arid1ab
MYCN
[55]
[82]
gas7
MYCN
[56]
2.2.4. Other Potentially Useful Methods
nestin
[83]
[84]
[85]
[86]
[87]
[88]
2.3. High Throughput Transplantation, Patient-Derived Xenograft (PDX) and In Vivo Drug Screening Using Zebrafish Larvae
To understand disease pathogenesis and screen or validate drug efficacy in vivo, scientists have successfully transplanted tumor cells with different genetic alteration(s) or manipulated gene expression, as well as patient-derived tumor cells into zebrafish at embryonic stage or adulthood [89]. Several features of zebrafish larvae make them uniquely suited for these studies, including: (i) transparent bodies that allow for easy tumor cell injection; (ii) ability to use trackable fluorescent-tagged cells following transplantation; (iii) an immature immune system during early embryonic development, which reduces the chance of the immune rejection of transplanted tumor cells; and (iv) availability of large clutches of embryos for transplantation. Multiple injection sites, such as the perivitelline space, pericardial space, yolk, retro-optical region, and brain, have been explored in a variety of studies to understand the mechanisms of tumor metastasis, angiogenesis, cellular intravasation/extravasation [90][91][92].
Drug screening on zebrafish transplants or xenografts is another exemplary usage of this model. Both embryos and adults can be used in high-throughput drug-screening assays. Embryos are relatively easy to work with due to their permeability of small molecules [93]. Researchers have already performed small-molecule drug screening using zebrafish embryos transplanted with neural crest stem cells (NCSCs) [52]. Since NB is derived from the sympathoadrenal lineage of neural crest cells, small molecules that inhibit NCSC induction might be potentially useful for the NB treatment. Among the 640 FDA-approved drugs applied in this screen, one drug, leflunomide, was identified to inhibit NCSC induction. Leflunomide, as an inhibitor of dihydroorotate dehydrogenase (DHODH) and an immunosuppressive agent for the treatment of patients with rheumatoid arthritis, has already been shown to reduce proliferation and induce apoptosis in NB cells both in vitro and in vivo [94]. Hence, this result further demonstrates the important application of zebrafish as an unbiased in vivo system for effective drug screening. Recently, zebrafish transplanted with human NB cells have been used to demonstrate the effect of a new multi-kinase drug, TP-0903, on reducing extravasation and inducing tumor cell death, suggesting the therapeutic potential of this compound for the NB treatment [51].
Although PDX mouse models are considered the gold standard for the in vivo validation of drug efficacy, the studies led by Drs. Ferreira and Fior, have demonstrated that the patient-derived zebrafish xenografts (zPDX, also called cancer “avatars”) can be used to sense cancer behavior and screen for potential novel therapies. Using a panel of zebrafish xenografts with patient-derived colorectal cancers, Ferreira and Fior rapidly screened the available therapeutic options for the colorectal cancers and predicted the treatment outcomes [92][95], which set the groundwork for using zPDX as a rapid in vivo screening platform for future personalized cancer treatments. Following these efforts, a high-throughput zebrafish xenograft assay of neuroblastoma was performed to confirm cannabinoid receptor 2 (CNR2) and Mitogen-activated protein kinase 8 (MAPK8) as promising candidates for the treatment of high-risk NB and to identify the drugs GW405833 and AS601245 as the most effective and well-tolerated CNR2 and MAPK8 targeted compounds to inhibit the growth of xenografts in zebrafish [96].
To better mimic the cytokine-enriched microenvironment found in human patients for xenotransplantation, Dr. Berman’s group generated the first humanized zebrafish by overexpressing transgenes encoding human hematopoietic-specific cytokines, such as GM-CSF, stem cell factor (SCF), or stromal cell-derived factor 1α (SDF1α). Transgenic lines with overexpression of each of the individual gene mentioned above were developed first using Tol2 transposon-mediated transgenic approach and then incrossed to generate a compound transgenic fish line with overexpression of all of the aforementioned cytokines (GM-CSF, SCF, and SDF1α) (designated GSS fish) [97]. Patient-derived leukemias transplanted into the GSS zebrafish exhibit improved survival, self-renewal ability and broader clonal representation. Therefore, the GSS fish establish a new standard for zebrafish xenotransplantation that more accurately recapitulates the human context for evaluating personalized treatment [97].
References
- Stanton, M.F. Diethylnitrosamine-Induced Hepatic Degeneration and Neoplasia in the Aquarium Fish, Brachydanio Rerio. J. Natl. Cancer Inst. 1965, 34, 117–130.
- Langenau, D.M.; Traver, D.; Ferrando, A.A.; Kutok, J.L.; Aster, J.C.; Kanki, J.P.; Lin, S.; Prochownik, E.; Trede, N.S.; Zon, L.I.; et al. Myc-induced T cell leukemia in transgenic zebrafish. Science 2003, 299, 887–890.
- Etchin, J.; Kanki, J.P.; Look, A.T. Zebrafish as a model for the study of human cancer. Methods Cell Biol. 2011, 105, 309–337.
- Feitsma, H.; Cuppen, E. Zebrafish as a cancer model. Mol. Cancer Res. 2008, 6, 685–694.
- Benjamin, D.C.; Hynes, R.O. Intravital imaging of metastasis in adult Zebrafish. BMC Cancer 2017, 17, 660.
- Kim, I.S.; Heilmann, S.; Kansler, E.R.; Zhang, Y.; Zimmer, M.; Ratnakumar, K.; Bowman, R.L.; Simon-Vermot, T.; Fennell, M.; Garippa, R.; et al. Microenvironment-derived factors driving metastatic plasticity in melanoma. Nat. Commun. 2017, 8, 14343.
- Colon, N.C.; Chung, D.H. Neuroblastoma. Adv. Pediatr. 2011, 58, 297–311.
- Maris, J.M. Recent advances in neuroblastoma. N. Engl. J. Med. 2010, 362, 2202–2211.
- Brodeur, G.M. Neuroblastoma: Biological insights into a clinical enigma. Nat. Rev. Cancer 2003, 3, 203–216.
- Pearson, A.D.; Pinkerton, C.R.; Lewis, I.J.; Imeson, J.; Ellershaw, C.; Machin, D. High-dose rapid and standard induction chemotherapy for patients aged over 1 year with stage 4 neuroblastoma: A randomised trial. Lancet Oncol. 2008, 9, 247–256.
- Proust-Houdemont, S.; Pasqualini, C.; Blanchard, P.; Dufour, C.; Benhamou, E.; Goma, G.; Semeraro, M.; Raquin, M.A.; Hartmann, O.; Valteau-Couanet, D. Busulfan-melphalan in high-risk neuroblastoma: The 30-year experience of a single institution. Bone Marrow Transplant. 2016, 51, 1076–1081.
- Yu, A.L.; Gilman, A.L.; Ozkaynak, M.F.; London, W.B.; Kreissman, S.G.; Chen, H.X.; Smith, M.; Anderson, B.; Villablanca, J.G.; Matthay, K.K.; et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 2010, 363, 1324–1334.
- Yu, A.L.; Gilman, A.L.; Ozkaynak, M.F.; Naranjo, A.; Diccianni, M.B.; Gan, J.; Hank, J.A.; Batova, A.; London, W.B.; Tenney, S.C.; et al. Long-term follow-up of a Phase III Study of ch14.18 (Dinutuximab) + Cytokine Immunotherapy in Children with High-risk Neuroblastoma: COG Study ANBL0032. Clin. Cancer Res. 2021.
- Basta, N.O.; Halliday, G.C.; Makin, G.; Birch, J.; Feltbower, R.; Bown, N.; Elliott, M.; Moreno, L.; Barone, G.; Pearson, A.D.; et al. Factors associated with recurrence and survival length following relapse in patients with neuroblastoma. Br. J. Cancer 2016, 115, 1048–1057.
- London, W.B.; Bagatell, R.; Weigel, B.J.; Fox, E.; Guo, D.; Van Ryn, C.; Naranjo, A.; Park, J.R. Historical time to disease progression and progression-free survival in patients with recurrent/refractory neuroblastoma treated in the modern era on Children’s Oncology Group early-phase trials. Cancer-Am. Cancer Soc. 2017, 123, 4914–4923.
- Schwab, M.; Alitalo, K.; Klempnauer, K.H.; Varmus, H.E.; Bishop, J.M.; Gilbert, F.; Brodeur, G.; Goldstein, M.; Trent, J. Amplified DNA with limited homology to myc cellular oncogene is shared by human neuroblastoma cell lines and a neuroblastoma tumour. Nature 1983, 305, 245–248.
- Huang, M.; Weiss, W.A. Neuroblastoma and MYCN. Cold Spring Harb. Perspect. Med. 2013, 3, a014415.
- Brodeur, G.M.; Seeger, R.C.; Schwab, M.; Varmus, H.E.; Bishop, J.M. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science 1984, 224, 1121–1124.
- Seeger, R.C.; Brodeur, G.M.; Sather, H.; Dalton, A.; Siegel, S.E.; Wong, K.Y.; Hammond, D. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N. Engl. J. Med. 1985, 313, 1111–1116.
- Schwab, M.; Varmus, H.E.; Bishop, J.M. Human N-myc gene contributes to neoplastic transformation of mammalian cells in culture. Nature 1985, 316, 160–162.
- Land, H.; Parada, L.F.; Weinberg, R.A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature 1983, 304, 596–602.
- Weiss, W.A.; Aldape, K.; Mohapatra, G.; Feuerstein, B.G.; Bishop, J.M. Targeted expression of MYCN causes neuroblastoma in transgenic mice. EMBO J. 1997, 16, 2985–2995.
- Berry, T.; Luther, W.; Bhatnagar, N.; Jamin, Y.; Poon, E.; Sanda, T.; Pei, D.; Sharma, B.; Vetharoy, W.R.; Hallsworth, A.; et al. The ALK(F1174L) mutation potentiates the oncogenic activity of MYCN in neuroblastoma. Cancer Cell 2012, 22, 117–130.
- Heukamp, L.C.; Thor, T.; Schramm, A.; De Preter, K.; Kumps, C.; De Wilde, B.; Odersky, A.; Peifer, M.; Lindner, S.; Spruessel, A.; et al. Targeted expression of mutated ALK induces neuroblastoma in transgenic mice. Sci. Transl. Med. 2012, 4, 141ra191.
- Cazes, A.; Lopez-Delisle, L.; Tsarovina, K.; Pierre-Eugene, C.; De Preter, K.; Peuchmaur, M.; Nicolas, A.; Provost, C.; Louis-Brennetot, C.; Daveau, R.; et al. Activated Alk triggers prolonged neurogenesis and Ret upregulation providing a therapeutic target in ALK-mutated neuroblastoma. Oncotarget 2014, 5, 2688–2702.
- Hennchen, M.; Stubbusch, J.; Abarchan-El Makhfi, I.; Kramer, M.; Deller, T.; Pierre-Eugene, C.; Janoueix-Lerosey, I.; Delattre, O.; Ernsberger, U.; Schulte, J.B.; et al. Lin28B and Let-7 in the Control of Sympathetic Neurogenesis and Neuroblastoma Development. J. Neurosci. 2015, 35, 16531–16544.
- Molenaar, J.J.; Domingo-Fernandez, R.; Ebus, M.E.; Lindner, S.; Koster, J.; Drabek, K.; Mestdagh, P.; van Sluis, P.; Valentijn, L.J.; van Nes, J.; et al. LIN28B induces neuroblastoma and enhances MYCN levels via let-7 suppression. Nat. Genet. 2012, 44, 1199–1206.
- Servenius, B.; Vernachio, J.; Price, J.; Andersson, L.C.; Peterson, P.A. Metastasizing neuroblastomas in mice transgenic for simian virus 40 large T (SV40T) under the olfactory marker protein gene promoter. Cancer Res. 1994, 54, 5198–5205.
- Iwakura, H.; Ariyasu, H.; Kanamoto, N.; Hosoda, K.; Nakao, K.; Kangawa, K.; Akamizu, T. Establishment of a novel neuroblastoma mouse model. Int. J. Oncol. 2008, 33, 1195–1199.
- Hattori, Y.; Kanamoto, N.; Kawano, K.; Iwakura, H.; Sone, M.; Miura, M.; Yasoda, A.; Tamura, N.; Arai, H.; Akamizu, T.; et al. Molecular characterization of tumors from a transgenic mouse adrenal tumor model: Comparison with human pheochromocytoma. Int. J. Oncol. 2010, 37, 695–705.
- Kamili, A.; Atkinson, C.; Trahair, T.N.; Fletcher, J.I. Mouse models of high-risk neuroblastoma. Cancer Metastasis Rev. 2020, 39, 261–274.
- Ornell, K.J.; Coburn, J.M. Developing preclinical models of neuroblastoma: Driving therapeutic testing. BMC Biomed. Eng. 2019, 1.
- Zhu, S.; Lee, J.S.; Guo, F.; Shin, J.; Perez-Atayde, A.R.; Kutok, J.L.; Rodig, S.J.; Neuberg, D.S.; Helman, D.; Feng, H.; et al. Activated ALK collaborates with MYCN in neuroblastoma pathogenesis. Cancer Cell 2012, 21, 362–373.
- Mosse, Y.P.; Laudenslager, M.; Longo, L.; Cole, K.A.; Wood, A.; Attiyeh, E.F.; Laquaglia, M.J.; Sennett, R.; Lynch, J.E.; Perri, P.; et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature 2008, 455, 930–935.
- Chen, Y.; Takita, J.; Choi, Y.L.; Kato, M.; Ohira, M.; Sanada, M.; Wang, L.; Soda, M.; Kikuchi, A.; Igarashi, T.; et al. Oncogenic mutations of ALK kinase in neuroblastoma. Nature 2008, 455, 971–974.
- Janoueix-Lerosey, I.; Lequin, D.; Brugieres, L.; Ribeiro, A.; de Pontual, L.; Combaret, V.; Raynal, V.; Puisieux, A.; Schleiermacher, G.; Pierron, G.; et al. Somatic and germline activating mutations of the ALK kinase receptor in neuroblastoma. Nature 2008, 455, 967–970.
- George, R.E.; Sanda, T.; Hanna, M.; Frohling, S.; Luther, W., 2nd; Zhang, J.; Ahn, Y.; Zhou, W.; London, W.B.; McGrady, P.; et al. Activating mutations in ALK provide a therapeutic target in neuroblastoma. Nature 2008, 455, 975–978.
- Carpenter, E.L.; Mosse, Y.P. Targeting ALK in neuroblastoma—Preclinical and clinical advancements. Nat. Rev. Clin. Oncol. 2012, 9, 391–399.
- Umapathy, G.; Mendoza-Garcia, P.; Hallberg, B.; Palmer, R.H. Targeting anaplastic lymphoma kinase in neuroblastoma. APMIS 2019, 127, 288–302.
- Amsterdam, A.; Lai, K.; Komisarczuk, A.Z.; Becker, T.S.; Bronson, R.T.; Hopkins, N.; Lees, J.A. Zebrafish Hagoromo mutants up-regulate fgf8 postembryonically and develop neuroblastoma. Mol. Cancer Res. 2009, 7, 841–850.
- Pei, D.; Luther, W.; Wang, W.; Paw, B.H.; Stewart, R.A.; George, R.E. Distinct neuroblastoma-associated alterations of PHOX2B impair sympathetic neuronal differentiation in zebrafish models. PLoS Genet. 2013, 9, e1003533.
- Brodeur, G.M.; Hogarty, M.; Bagatell, R.; Mosse, Y.P.; Maris, J.M. Neuroblastoma. In Principles and Practice of Pediatric Oncology, 7th ed.; Pizzo, P.A., Poplack, D., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2016; pp. 772–792.
- Pinto, N.R.; Applebaum, M.A.; Volchenboum, S.L.; Matthay, K.K.; London, W.B.; Ambros, P.F.; Nakagawara, A.; Berthold, F.; Schleiermacher, G.; Park, J.R.; et al. Advances in Risk Classification and Treatment Strategies for Neuroblastoma. J. Clin. Oncol. 2015, 33, 3008–3017.
- He, S.; Mansour, M.R.; Zimmerman, M.W.; Ki, D.H.; Layden, H.M.; Akahane, K.; Gjini, E.; de Groh, E.D.; Perez-Atayde, A.R.; Zhu, S.; et al. Synergy between loss of NF1 and overexpression of MYCN in neuroblastoma is mediated by the GAP-related domain. Elife 2016, 5.
- Zhang, X.; Dong, Z.; Zhang, C.; Ung, C.Y.; He, S.; Tao, T.; Oliveira, A.M.; Meves, A.; Ji, B.; Look, A.T.; et al. Critical Role for GAB2 in Neuroblastoma Pathogenesis through the Promotion of SHP2/MYCN Cooperation. Cell Rep. 2017, 18, 2932–2942.
- Zhu, S.; Zhang, X.; Weichert-Leahey, N.; Dong, Z.; Zhang, C.; Lopez, G.; Tao, T.; He, S.; Wood, A.C.; Oldridge, D.; et al. LMO1 Synergizes with MYCN to Promote Neuroblastoma Initiation and Metastasis. Cancer Cell 2017, 32, 310–323.e315.
- Radic-Sarikas, B.; Halasz, M.; Huber, K.V.M.; Winter, G.E.; Tsafou, K.P.; Papamarkou, T.; Brunak, S.; Kolch, W.; Superti-Furga, G. Lapatinib potentiates cytotoxicity of YM155 in neuroblastoma via inhibition of the ABCB1 efflux transporter. Sci. Rep. 2017, 7, 3091.
- Tao, T.; Sondalle, S.B.; Shi, H.; Zhu, S.; Perez-Atayde, A.R.; Peng, J.; Baserga, S.J.; Look, A.T. The pre-rRNA processing factor DEF is rate limiting for the pathogenesis of MYCN-driven neuroblastoma. Oncogene 2017, 36, 3852–3867.
- Zimmerman, M.W.; Liu, Y.; He, S.; Durbin, A.D.; Abraham, B.J.; Easton, J.; Shao, Y.; Xu, B.; Zhu, S.; Zhang, X.; et al. MYC Drives a Subset of High-Risk Pediatric Neuroblastomas and Is Activated through Mechanisms Including Enhancer Hijacking and Focal Enhancer Amplification. Cancer Discov. 2018, 8, 320–335.
- Shen, J.; Najafi, S.; Stable, S.; Fabian, J.; Koeneke, E.; Kolbinger, F.R.; Wrobel, J.K.; Meder, B.; Distel, M.; Heimburg, T.; et al. A kinome-wide RNAi screen identifies ALK as a target to sensitize neuroblastoma cells for HDAC8-inhibitor treatment. Cell Death Differ. 2018, 25, 2053–2070.
- Aveic, S.; Corallo, D.; Porcu, E.; Pantile, M.; Boso, D.; Zanon, C.; Viola, G.; Sidarovich, V.; Mariotto, E.; Quattrone, A.; et al. TP-0903 inhibits neuroblastoma cell growth and enhances the sensitivity to conventional chemotherapy. Eur. J. Pharmacol. 2018, 818, 435–448.
- Seda, M.; Geerlings, M.; Lim, P.; Jeyabalan-Srikaran, J.; Cichon, A.C.; Scambler, P.J.; Beales, P.L.; Hernandez-Hernandez, V.; Stoker, A.W.; Jenkins, D. An FDA-Approved Drug Screen for Compounds Influencing Craniofacial Skeletal Development and Craniosynostosis. Mol. Syndromol. 2019, 10, 98–114.
- Koach, J.; Holien, J.K.; Massudi, H.; Carter, D.R.; Ciampa, O.C.; Herath, M.; Lim, T.; Seneviratne, J.A.; Milazzo, G.; Murray, J.E.; et al. Drugging MYCN Oncogenic Signaling through the MYCN-PA2G4 Binding Interface. Cancer Res. 2019, 79, 5652–5667.
- Tao, T.; Shi, H.; Mariani, L.; Abraham, B.J.; Durbin, A.D.; Zimmerman, M.W.; Powers, J.T.; Missios, P.; Ross, K.N.; Perez-Atayde, A.R.; et al. LIN28B regulates transcription and potentiates MYCN-induced neuroblastoma through binding to ZNF143 at target gene promotors. Proc. Natl. Acad. Sci. USA 2020, 117, 16516–16526.
- Shi, H.; Tao, T.; Abraham, B.J.; Durbin, A.D.; Zimmerman, M.W.; Kadoch, C.; Look, A.T. ARID1A loss in neuroblastoma promotes the adrenergic-to-mesenchymal transition by regulating enhancer-mediated gene expression. Sci. Adv. 2020, 6, eaaz3440.
- Dong, Z.; Yeo, K.S.; Lopez, G.; Zhang, C.; Dankert Eggum, E.N.; Rokita, J.L.; Ung, C.Y.; Levee, T.M.; Her, Z.P.; Howe, C.J.; et al. GAS7 Deficiency Promotes Metastasis in MYCN-driven Neuroblastoma. Cancer Res. 2021.
- Corallo, D.; Candiani, S.; Ori, M.; Aveic, S.; Tonini, G.P. The zebrafish as a model for studying neuroblastoma. Cancer Cell Int. 2016, 16, 82.
- Casey, M.J.; Stewart, R.A. Zebrafish as a model to study neuroblastoma development. Cell Tissue Res. 2018, 372, 223–232.
- Quarta, C.; Cantelli, E.; Nanni, C.; Ambrosini, V.; D’Ambrosio, D.; Di Leo, K.; Angelucci, S.; Zagni, F.; Lodi, F.; Marengo, M.; et al. Molecular imaging of neuroblastoma progression in TH-MYCN transgenic mice. Mol. Imaging Biol. 2013, 15, 194–202.
- Almeida, G.S.; Panek, R.; Hallsworth, A.; Webber, H.; Papaevangelou, E.; Boult, J.K.; Jamin, Y.; Chesler, L.; Robinson, S.P. Pre-clinical imaging of transgenic mouse models of neuroblastoma using a dedicated 3-element solenoid coil on a clinical 3T platform. Br. J. Cancer 2017, 117, 791–800.
- Teitelman, G.; Baker, H.; Joh, T.H.; Reis, D.J. Appearance of catecholamine-synthesizing enzymes during development of rat sympathetic nervous system: Possible role of tissue environment. Proc. Natl. Acad. Sci. USA 1979, 76, 509–513.
- Marusich, M.F.; Furneaux, H.M.; Henion, P.D.; Weston, J.A. Hu neuronal proteins are expressed in proliferating neurogenic cells. J. Neurobiol. 1994, 25, 143–155.
- Dong, R.; Yang, R.; Zhan, Y.; Lai, H.D.; Ye, C.J.; Yao, X.Y.; Luo, W.Q.; Cheng, X.M.; Miao, J.J.; Wang, J.F.; et al. Single-Cell Characterization of Malignant Phenotypes and Developmental Trajectories of Adrenal Neuroblastoma. Cancer Cell 2020, 38, 716–733.e716.
- Hsu, H.J.; Lin, G.; Chung, B.C. Parallel early development of zebrafish interrenal glands and pronephros: Differential control by wt1 and ff1b. Development 2003, 130, 2107–2116.
- Renshaw, S.A.; Trede, N.S. A model 450 million years in the making: Zebrafish and vertebrate immunity. Dis. Model. Mech. 2012, 5, 38–47.
- Lieschke, G.J.; Trede, N.S. Fish immunology. Curr. Biol. 2009, 19, R678–R682.
- Menke, A.L.; Spitsbergen, J.M.; Wolterbeek, A.P.; Woutersen, R.A. Normal anatomy and histology of the adult zebrafish. Toxicol. Pathol. 2011, 39, 759–775.
- Progatzky, F.; Cook, H.T.; Lamb, J.R.; Bugeon, L.; Dallman, M.J. Mucosal inflammation at the respiratory interface: A zebrafish model. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 310, L551–L561.
- DuBois, S.G.; Kalika, Y.; Lukens, J.N.; Brodeur, G.M.; Seeger, R.C.; Atkinson, J.B.; Haase, G.M.; Black, C.T.; Perez, C.; Shimada, H.; et al. Metastatic sites in stage IV and IVS neuroblastoma correlate with age, tumor biology, and survival. J. Pediatr. Hematol. Oncol. 1999, 21, 181–189.
- Levental, K.R.; Yu, H.; Kass, L.; Lakins, J.N.; Egeblad, M.; Erler, J.T.; Fong, S.F.; Csiszar, K.; Giaccia, A.; Weninger, W.; et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 2009, 139, 891–906.
- Paszek, M.J.; Zahir, N.; Johnson, K.R.; Lakins, J.N.; Rozenberg, G.I.; Gefen, A.; Reinhart-King, C.A.; Margulies, S.S.; Dembo, M.; Boettiger, D.; et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 2005, 8, 241–254.
- Luo, C.; Zuniga, J.; Edison, E.; Palla, S.; Dong, W.; Parker-Thornburg, J. Superovulation strategies for 6 commonly used mouse strains. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 471–478.
- Gaiano, N.; Amsterdam, A.; Kawakami, K.; Allende, M.; Becker, T.; Hopkins, N. Insertional mutagenesis and rapid cloning of essential genes in zebrafish. Nature 1996, 383, 829–832.
- Allende, M.L.; Amsterdam, A.; Becker, T.; Kawakami, K.; Gaiano, N.; Hopkins, N. Insertional mutagenesis in zebrafish identifies two novel genes, pescadillo and dead eye, essential for embryonic development. Genes Dev. 1996, 10, 3141–3155.
- Thermes, V.; Grabher, C.; Ristoratore, F.; Bourrat, F.; Choulika, A.; Wittbrodt, J.; Joly, J.S. I-SceI meganuclease mediates highly efficient transgenesis in fish. Mech. Dev. 2002, 118, 91–98.
- Pan, F.C.; Chen, Y.; Loeber, J.; Henningfeld, K.; Pieler, T. I-SceI meganuclease-mediated transgenesis in Xenopus. Dev. Dyn. 2006, 235, 247–252.
- Powers, J.T.; Tsanov, K.M.; Pearson, D.S.; Roels, F.; Spina, C.S.; Ebright, R.; Seligson, M.; de Soysa, Y.; Cahan, P.; Theissen, J.; et al. Multiple mechanisms disrupt the let-7 microRNA family in neuroblastoma. Nature 2016, 535, 246–251.
- Hwang, W.Y.; Fu, Y.; Reyon, D.; Maeder, M.L.; Tsai, S.Q.; Sander, J.D.; Peterson, R.T.; Yeh, J.R.; Joung, J.K. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat. Biotechnol. 2013, 31, 227–229.
- Jao, L.E.; Wente, S.R.; Chen, W. Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proc. Natl. Acad. Sci. USA 2013, 110, 13904–13909.
- Varshney, G.K.; Pei, W.; LaFave, M.C.; Idol, J.; Xu, L.; Gallardo, V.; Carrington, B.; Bishop, K.; Jones, M.; Li, M.; et al. High-throughput gene targeting and phenotyping in zebrafish using CRISPR/Cas9. Genome Res. 2015, 25, 1030–1042.
- Hruscha, A.; Krawitz, P.; Rechenberg, A.; Heinrich, V.; Hecht, J.; Haass, C.; Schmid, B. Efficient CRISPR/Cas9 genome editing with low off-target effects in zebrafish. Development 2013, 140, 4982–4987.
- Bedell, V.M.; Wang, Y.; Campbell, J.M.; Poshusta, T.L.; Starker, C.G.; Krug, R.G., 2nd; Tan, W.; Penheiter, S.G.; Ma, A.C.; Leung, A.Y.; et al. In vivo genome editing using a high-efficiency TALEN system. Nature 2012, 491, 114–118.
- Seok, S.H.; Na, Y.R.; Han, J.H.; Kim, T.H.; Jung, H.; Lee, B.H.; Emelyanov, A.; Parinov, S.; Park, J.H. Cre/loxP-regulated transgenic zebrafish model for neural progenitor-specific oncogenic Kras expression. Cancer Sci. 2010, 101, 149–154.
- Langenau, D.M.; Feng, H.; Berghmans, S.; Kanki, J.P.; Kutok, J.L.; Look, A.T. Cre/lox-regulated transgenic zebrafish model with conditional myc-induced T cell acute lymphoblastic leukemia. Proc. Natl. Acad. Sci. USA 2005, 102, 6068–6073.
- Robles, E.; Filosa, A.; Baier, H. Precise lamination of retinal axons generates multiple parallel input pathways in the tectum. J. Neurosci. 2013, 33, 5027–5039.
- Felker, A.; Mosimann, C. Contemporary zebrafish transgenesis with Tol2 and application for Cre/lox recombination experiments. Methods Cell Biol. 2016, 135, 219–244.
- Burg, L.; Palmer, N.; Kikhi, K.; Miroshnik, E.S.; Rueckert, H.; Gaddy, E.; MacPherson Cunningham, C.; Mattonet, K.; Lai, S.L.; Marin-Juez, R.; et al. Conditional mutagenesis by oligonucleotide-mediated integration of loxP sites in zebrafish. PLoS Genet. 2018, 14, e1007754.
- Carney, T.J.; Mosimann, C. Switch and Trace: Recombinase Genetics in Zebrafish. Trends Genet. 2018, 34, 362–378.
- Veinotte, C.J.; Dellaire, G.; Berman, J.N. Hooking the big one: The potential of zebrafish xenotransplantation to reform cancer drug screening in the genomic era. Dis. Model. Mech. 2014, 7, 745–754.
- Haney, M.G.; Moore, L.H.; Blackburn, J.S. Drug Screening of Primary Patient Derived Tumor Xenografts in Zebrafish. J. Vis. Exp. 2020, e60996.
- Cabezas-Sainz, P.; Pensado-Lopez, A.; Sainz, B., Jr.; Sanchez, L. Modeling Cancer Using Zebrafish Xenografts: Drawbacks for Mimicking the Human Microenvironment. Cells 2020, 9, 1978.
- Costa, B.; Estrada, M.F.; Mendes, R.V.; Fior, R. Zebrafish Avatars towards Personalized Medicine-A Comparative Review between Avatar Models. Cells 2020, 9, 293.
- Pichler, F.B.; Laurenson, S.; Williams, L.C.; Dodd, A.; Copp, B.R.; Love, D.R. Chemical discovery and global gene expression analysis in zebrafish. Nat. Biotechnol. 2003, 21, 879–883.
- Zhu, S.; Yan, X.; Xiang, Z.; Ding, H.F.; Cui, H. Leflunomide reduces proliferation and induces apoptosis in neuroblastoma cells in vitro and in vivo. PLoS ONE 2013, 8, e71555.
- Fior, R.; Povoa, V.; Mendes, R.V.; Carvalho, T.; Gomes, A.; Figueiredo, N.; Ferreira, M.G. Single-cell functional and chemosensitive profiling of combinatorial colorectal therapy in zebrafish xenografts. Proc. Natl. Acad. Sci. USA 2017, 114, E8234–E8243.
- Almstedt, E.; Elgendy, R.; Hekmati, N.; Rosen, E.; Warn, C.; Olsen, T.K.; Dyberg, C.; Doroszko, M.; Larsson, I.; Sundstrom, A.; et al. Integrative discovery of treatments for high-risk neuroblastoma. Nat. Commun. 2020, 11, 71.
- Rajan, V.; Melong, N.; Hing Wong, W.; King, B.; Tong, S.R.; Mahajan, N.; Gaston, D.; Lund, T.; Rittenberg, D.; Dellaire, G.; et al. Humanized zebrafish enhance human hematopoietic stem cell survival and promote acute myeloid leukemia clonal diversity. Haematologica 2020, 105, 2391–2399.
