Cancer is a major burden of disease globally. Each year, tens of millions of people are diagnosed with cancer worldwide, and more than half of the patients eventually die from it. Significant advances have been noticed in cancer treatment, but the mortality and incidence rates of cancers are still high. Thus, there is a growing research interest in developing more effective and less toxic cancer treatment approaches. Curcumin (CUR), the major active component of turmeric (Curcuma longa L.), has gained great research interest as an antioxidant, anticancer, and anti-inflammatory agent. This natural compound shows its anticancer effect through several pathways including interfering with multiple cellular mechanisms and inhibiting/inducing the generation of multiple cytokines, enzymes, or growth factors including IκB kinase β (IκKβ), tumor necrosis factor-alpha (TNF-α), signal transducer, and activator of transcription 3 (STAT3), cyclooxygenase II (COX-2), protein kinase D1 (PKD1), nuclear factor-kappa B (NF-κB), epidermal growth factor, and mitogen-activated protein kinase (MAPK). Interestingly, the anticancer activity of CUR has been limited primarily due to its poor water solubility, which can lead to low chemical stability, low oral bioavailability, and low cellular uptake. Delivering drugs at a controlled rate, slow delivery, and targeted delivery are other very attractive methods and have been pursued vigorously.
- Curcuma longa
- curcumin
- anticancer
- mechanism of action
- cellular mechanisms
1. Introduction
Globally, cancer is the second leading cause of death and is one of the main causes of public health problems. In 2018 alone, there were about 1.73 million new cancer cases and over 609,000 cancer-related deaths in the United States
[1]
. Although there are some noticeable advances in cancer treatment, the occurrence of cancer and mortality rate has not decreased in the last 30 years
[2]
. In the case of treatment and prevention of cancer, improved knowledge regarding molecular changes that contribute to the development and advancement of cancer is crucial. Various common approaches can be used to target specific cancer cells in order to suppress the development of tumor, metastasis, and progression without exerting serious side effects
[3]
. Along with the chemically synthesized anticancer drugs, various anticancer agents have been extracted from several plants including
Curcuma longa (C. longa)
,
Erythroxylumprevillei
,
Cephalotaxus
species,
Betula alba
,
Catharanthusroseus
, and
Taxusbrevifolia
. Furthermore, it has been demonstrated that numerous plant species exhibit anti-cancer properties and there is a growing interest regarding these plants, particularly in developing countries
.
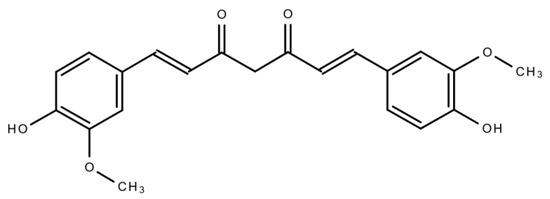
Curcumin (CUR) (
) is a polyphenolic compound extracted from
C. longa
(turmeric) rhizomes. In 1815, this compound was first isolated by two scientists, namely, Pelletier and Vogel
[11]
. Following this discovery, there was a growing research interest regarding CUR, which led to the identification of the numerous health benefits of CUR. This polyphenolic compound is also familiar as diferuloylmethane. Its molecular weight is 368.38 and its chemical formula is C
21
H
20
O
6
[12]
. CUR has shown its activity against multiple chronic diseases including neurodegenerative disorders, obesity, liver disease, metabolic syndrome, arthritis, inflammation, and multiple cancers
. Indeed, CUR is a highly effective candidate in cancer treatment as a single-drug therapy or in combination with other therapeutic agents.This natural compound also has the capacity to influence various molecular targets and signaling mechanisms that are linked with various cancers
.
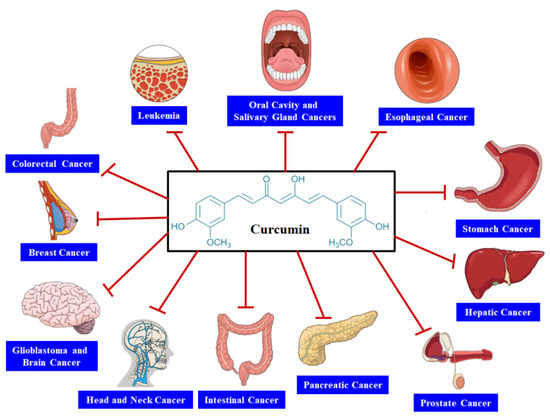
In this article, we have summarized CUR’s anticancer activity against several cancers such as gastrointestinal, brain, head and neck, pancreatic, prostate, breast, and colorectal cancers. Furthermore, we also focused on the findings obtained from several experimental and clinical studies regarding the anticancer action of CUR in animal models, human subjects, and cancer cell lines.
2. Mechanism of Action of Curcumin as an Anticancer Agent
An imbalance between cell death and cell proliferation is regarded as one of the major causal factors of cancer
[17]
. Uncontrolled cell proliferation is likely to occur if the cells skip death, which can lead to various types of cancers
[18]
. The intrinsic and extrinsic pathways are responsible for generating apoptotic signals. It has been found that the intrinsic pathway plays a role via inducing the mitochondrial membrane to suppress the expressions of B-cell lymphoma-extra large and B-cell lymphoma 2 (Bcl-2)
[19]
. CUR has the ability to disrupt the balance of mitochondrial membrane potential, which can result in increased Bcl-xL suppression
[20]
. On the other hand, the extrinsic apoptotic pathway functions via inducing the tumor necrosis factor (TNF)-associated apoptosis and elevating the death receptors (DRs) on cells. In this pathway, CUR plays a role via upregulating DR4 and DR expression
. It has been revealed by in vitro studies that CUR and its derivatives can excellently stimulate apoptosis in various cell lines through downregulating or suppressing intracellular transcription factors. These transcription factors include matrix metalloproteinase-9 (MMP-9), signal transducer and activator of transcription 3 (STAT3), cyclooxygenase II (COX-2), activator protein 1 (AP-1), nuclear factor-kappa B (NF-κB), and nitric oxide synthase
. CUR can also exert its anticancer effect via reducing lactate production and uptake of glucose in cancer cells through pyruvate kinase M2 (PKM2)downregulation. PKM2 suppression was found to be attained by inhibiting the mammalian target of rapamycin-hypoxia-inducible factor 1α (mTOR-HIF1α)
[26]
. Multiple analyses have examined the capacity of CUR and derivatives of CUR to inhibit several types of cancers through interacting with various molecular targets (
).
In CL-5 xenograft tumors, CUR can trigger apoptosis and caused downregulation of cyclin D1, c-Met, Akt, and epidermal growth factor receptor (EGFR)
[27]
. Furthermore, CUR suppressed metastasis and lung cell invasion via upregulating the expression of HLJ1 in cancer cells
[28]
. Along with the activity of CUR on nuclear factor-κB (NF-κB) and STAT3 signaling cascades, CUR also suppressed cell cycle arrest and cell proliferation and induced apoptosis by modulating other transcription factors including PPAR-α, Hif-1, Notch-1, β-catenin, p53, Erg-1, and AP-1
[29]
. It has been confirmed that CUR suppressed the phosphorylation of focal adhesion kinase (FAK) and increased the expression of multiple extracellular matrix (ECM) components, which further contribute to metastasis and invasion. In a concentration-dependent manner, CUR also increased cell adhesion via inducing various ECM components including fibronectin, laminin, collagen IX, collagen IV, collagen III, and collagen I. Collectively, these findings have indicated that CUR inhibits FAK action via suppression of its phosphorylation sites and triggers ECM components to improve cell adhesion, which can eventually prevent cell migration and detachment of cancer cells. It was reported that suppression of FAK expression resulted in elevated cell adhesion, which eventually playeda role in the anti-invasive activity of CUR
[30]
. In colorectal cancer cells, CUR decreased the expression of CD24 in a dose-dependent manner. In addition, expression of E-cadherin was elevated by CUR and played a role as a suppressor of epithelial-mesenchymal transition. In colorectal cancer cells, CUR may exhibit its action against metastasis by downregulating CD24, FAK, and Sp-1, and upregulating the expression of E-cadherin
[30]
. In a study, Zhou et al.
[30]
assessed the activity of 11 CUR-associated compounds (comprising a benzyl piperidone moiety) in various cancer cell lines. Furthermore, they observed that some of these compounds decreased the level of the phospho-extracellular signal-regulated kinase (Erk)1/2 and phospho-Akt
[30]
. It has been reported that autophagy and ER stress might have a significant contribution in the case of apoptosis, which is triggered via the CUR analogue B19 in hepatocellular carcinoma cells and the epithelial ovarian tumor cell line, and that suppression of autophagy may elevate CUR analogue-triggered apoptosis via stimulating severe ER stress. In ovarian cancer cell lines, this CUR analogue might also induce apoptosis, autophagy, and ER stress in vitro
. It was confirmed that autophagy may play role in programmed cell death type II and might effectively inhibit the growth of malignant glioma cells after treatment with CUR
[33]
.
3. Bioavailability of Curcumin
CUR is safer to use and it has great potential as an anticancer agent. However, its major drawback is its poor oral bioavailability, which takes place because of its substantial first-pass effect and low aqueous solubility
. At tumor sites, increased permeability and retention action of nanomaterials might ameliorate the buildup of chemotherapeutic agents. In this regard, for instance, micelles, dendrimers, carbon nanotubes, and liposomes, have been utilized as carriers for cisplatin, paclitaxel, doxorubicin, and SN38 in order to decrease adverse effects and improve concentrations of drugs in tumors
. Increased solubility of chemotherapeutic drugs is another benefit of utilizing nanomaterials as drug carriers. There is a growing interest in self-assembling peptide nanofibers due to their easy modification, good biocompatibility, and design flexibility by a “bottom-up” technique
. These nanofibers have been extensively utilized in several cell cultures and also in drug delivery systems to decrease adverse effects, ameliorate buildup at the tumor site, and improve the solubility of a hydrophobic drug
[47]
. The enhanced anticancer activity has been observed with the nanofiber-encapsulated antitumor agents including ellipticine, camptothecin, and paclitaxel
. Several studies have confirmed that as drug carriers, the 2-dimensional structure of peptide nanofibers is much better compared to the 3-dimensional structure of nanoparticles. In a study, Wagh et al.
[51]
showed that peptide-based nanofibershada rapid elimination rate, improved tumor targeting within a shorter period, and better biocompatibility compared to carbon rods, and spherical nanomaterials (selenium and cadmium quantum dots, poly(lactic-co-glycolic acid) or PLGA, cadmium, gold, and polystyrene).
CUR has been widely studied and several synthetic analogues of CUR have been generated and analyzed for potential therapeutic effects
[52][53][54][55][56][57][58][59]
. Some of these analogues showed excellent actions in several cancer animal models and cell lines. It has been revealed by studies that CUR-associated compounds containing benzyl piperidone possess increased biological effects and absorption
. In addition, studies have also confirmed effective anticancer activities of CUR analogues
. The introduction of CUR into nano-formulations to increase water-solubility has outstandingly transformed its bioavailability. Moreover, nano-formulations have enhanced the transport and improved in vitro CUR levels in the cell, whereas their extended-release formulas as well as their increased compatibility appear to be excellent for their in vivo activities
.
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA. Cancer J. Clin. 2018, 68, 7–30.
- Gupta, A.P.; Pandotra, P.; Sharma, R.; Kushwaha, M.; Gupta, S. Marine resource: A promising future for anticancer drugs. In Studies in Natural Products Chemistry; Elsevier B.V.: Amsterdam, The Netherlands, 2013; Volume 40, pp. 229–325. ISBN 9780444596031.
- Umar, A.; Dunn, B.K.; Greenwald, P. Future directions in cancer prevention. Nat. Rev. Cancer 2012, 12, 835–848.
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A review of curcumin and its derivatives as anticancer agents. Int. J. Mol. Sci. 2019, 20, 1033.
- Ochwang’i, D.O.; Kimwele, C.N.; Oduma, J.A.; Gathumbi, P.K.; Mbaria, J.M.; Kiama, S.G. Medicinal plants used in treatment and management of cancer in Kakamega County, Kenya. J. Ethnopharmacol. 2014, 151, 1040–1055.
- Freiburghaus, F.; Kaminsky, R.; Nkunya, M.H.H.; Brun, R. Evaluation of African medicinal plants for their in vitro trypanocidal activity. J. Ethnopharmacol. 1996, 55, 1–11.
- Costa-Lotufo, L.V.; Khan, M.T.H.; Ather, A.; Wilke, D.V.; Jimenez, P.C.; Pessoa, C.; De Moraes, M.E.A.; De Moraes, M.O. Studies of the anticancer potential of plants used in Bangladeshi folk medicine. J. Ethnopharmacol. 2005, 99, 21–30.
- Cai, Y.Z.; Sun, M.; Xing, J.; Luo, Q.; Corke, H. Structure-radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006, 78, 2872–2888.
- Fouche, G.; Cragg, G.M.; Pillay, P.; Kolesnikova, N.; Maharaj, V.J.; Senabe, J. In vitro anticancer screening of South African plants. J. Ethnopharmacol. 2008, 119, 455–461.
- Kamatou, G.P.P.; Van Zyl, R.L.; Davids, H.; Van Heerden, F.R.; Lourens, A.C.U.; Viljoen, A.M. Antimalarial and anticancer activities of selected South African Salvia species and isolated compounds from S. radula. South African J. Bot. 2008, 74, 238–243.
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin. Exp. Pharmacol. Physiol. 2012, 39, 283–299.
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112.
- He, Y.; Yue, Y.; Zheng, X.; Zhang, K.; Chen, S.; Du, Z. Curcumin, inflammation, and chronic diseases: How are they linked? Molecules 2015, 20, 9183–9213.
- Hewlings, S.; Kalman, D. Curcumin: A Review of Its’ Effects on Human Health. Foods 2017, 6, 92.
- Giordano, A.; Tommonaro, G. Curcumin and cancer. Nutrients 2019, 11, 2376.
- Tan, B.L.; Norhaizan, M.E. Curcumin combination chemotherapy: The implication and efficacy in cancer. Molecules 2019, 24, 2527.
- Wong, R.S.Y. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87.
- Bauer, J.H.; Helfand, S.L. New tricks of an old molecule: Lifespan regulation by p53. Aging Cell 2006, 5, 437–440.
- Tuorkey, M. Curcumin a potent cancer preventive agent: Mechanisms of cancer cell killing. Interv. Med. Appl. Sci. 2014, 6, 139–146.
- Balasubramanian, S.; Eckert, R.L. Curcumin suppresses AP1 transcription factor-dependent differentiation and activates apoptosis in human epidermal keratinocytes. J. Biol. Chem. 2007, 282, 6707–6715.
- Moragoda, L.; Jaszewski, R.; Majumdar, A.P.N. Curcumin induced modulation of cell cycle and apoptosis in gastric and colon cancer cells. Anticancer Res. 2001, 21, 873–878.
- Ashour, A.A.; Abdel-Aziz, A.A.H.; Mansour, A.M.; Neslihan Alpay, S.; Huo, L.; Ozpolat, B. Targeting elongation factor-2 kinase (eEF-2K) induces apoptosis in human pancreatic cancer cells. Apoptosis 2014, 19, 241–258.
- Lee, H.P.; Li, T.M.; Tsao, J.Y.; Fong, Y.C.; Tang, C.H. Curcumin induces cell apoptosis in human chondrosarcoma through extrinsic death receptor pathway. Int. Immunopharmacol. 2012, 13, 163–169.
- Hahn, Y.I.; Kim, S.J.; Choi, B.Y.; Cho, K.C.; Bandu, R.; Kim, K.P.; Kim, D.H.; Kim, W.; Park, J.S.; Han, B.W.; et al. Curcumin interacts directly with the Cysteine 259 residue of STAT3 and induces apoptosis in H-Ras transformed human mammary epithelial cells. Sci. Rep. 2018, 8, 6409.
- Lee, W.H.; Loo, C.Y.; Young, P.M.; Traini, D.; Mason, R.S.; Rohanizadeh, R. Recent advances in curcumin nanoformulation for cancer therapy. Expert Opin. Drug Deliv. 2014, 11, 1183–1201.
- Siddiqui, F.A.; Prakasam, G.; Chattopadhyay, S.; Rehman, A.U.; Padder, R.A.; Ansari, M.A.; Irshad, R.; Mangalhara, K.; Bamezai, R.N.K.; Husain, M.; et al. Curcumin decreases Warburg effect in cancer cells by down-regulating pyruvate kinase M2 via mTOR-HIF1α inhibition. Sci. Rep. 2018, 8, 8323.
- Lee, J.Y.; Lee, Y.M.; Chang, G.C.; Yu, S.L.; Hsieh, W.Y.; Chen, J.J.W.; Chen, H.W.; Yang, P.C. Curcumin induces EGFR degradation in lung adenocarcinoma and modulates p38 activation in intestine: The versatile adjuvant for gefitinib therapy. PLoS ONE 2011, 6, e23756.
- Shishodia, S.; Singh, T.; Chaturvedi, M.M. Modulation of transcription factors by curcumin. Adv. Exp. Med. Biol. 2007, 595, 127–148.
- Davie, J.R.; He, S.; Li, L.; Sekhavat, A.; Espino, P.; Drobic, B.; Dunn, K.L.; Sun, J.M.; Chen, H.Y.; Yu, J.; et al. Nuclear organization and chromatin dynamics - Sp1, Sp3 and histone deacetylases. Adv. Enzyme Regul. 2008, 48, 189–208.
- Zhou, D.-Y.; Zhang, K.; Conney, A.H.; Ding, N.; Cui, X.-X.; Wang, H.; Verano, M.; Zhao, S.; Fan, Y.-X.; Zheng, X.; et al. Synthesis and Evaluation of Curcumin-Related Compounds Containing Benzyl Piperidone for Their Effects on Human Cancer Cells. Chem. Pharm. Bull. 2013, 61, 1149–1155.
- Grabacka, M.M.; Gawin, M.; Pierzchalska, M. Phytochemical modulators of mitochondria: The search for chemopreventive agents and supportive therapeutics. Pharmaceuticals 2014, 7, 913–942.
- Zhou, T.; Ye, L.; Bai, Y.; Sun, A.; Cox, B.; Liu, D.; Li, Y.; Liotta, D.; Snyder, J.P.; Fu, H.; et al. Autophagy and Apoptosis in Hepatocellular Carcinoma Induced by EF25-(GSH)2: A Novel Curcumin Analog. PLoS ONE 2014, 9, e107876.
- Aoki, H.; Takada, Y.; Kondo, S.; Sawaya, R.; Aggarwal, B.B.; Kondo, Y. Evidence that curcumin suppresses the growth of malignant gliomas in vitro and in vivo through induction of autophagy: Role of akt and extracellular signal-regulated kinase signaling pathways. Mol. Pharmacol. 2007, 72, 29–39.
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008, 14, 4491–4499.
- Zlotogorski, A.; Dayan, A.; Dayan, D.; Chaushu, G.; Salo, T.; Vered, M. Nutraceuticals as new treatment approaches for oral cancer-I: Curcumin. Oral Oncol. 2013, 49, 187–191.
- Adams, B.K.; Ferstl, E.M.; Davis, M.C.; Herold, M.; Kurtkaya, S.; Camalier, R.F.; Hollingshead, M.G.; Kaur, G.; Sausville, E.A.; Rickles, F.R.; et al. Synthesis and biological evaluation of novel curcumin analogs as anti-cancer and anti-angiogenesis agents. Bioorganic Med. Chem. 2004, 12, 3871–3883.
- Lee, K.H.; Farida, F.H.; Syahida, A.; Abas, F.; Shaari, K.; Israf, D.A.; Lajis, N.H. Synthesis and biological evaluation of curcumin-like diarylpentanoid analogues for anti-inflammatory, antioxidant and anti-tyrosinase activities. Eur. J. Med. Chem. 2009, 44, 3195–3200.
- Zhao, C.; Yang, J.; Wang, Y.; Liang, D.; Yang, X.; Li, X.; Wu, J.; Wu, X.; Yang, S.; Li, X.; et al. Synthesis of mono-carbonyl analogues of curcumin and their effects on inhibition of cytokine release in LPS-stimulated RAW 264.7 macrophages. Bioorg. Med. Chem. 2010, 18, 2388–2393.
- Bala, V.; Rao, S.; Boyd, B.J.; Prestidge, C.A. Prodrug and nanomedicine approaches for the delivery of the camptothecin analogue SN38. J. Control. Release 2013, 172, 48–61.
- Teow, H.M.; Zhou, Z.; Najlah, M.; Yusof, S.R.; Abbott, N.J.; D’Emanuele, A. Delivery of paclitaxel across cellular barriers using a dendrimer-based nanocarrier. Int. J. Pharm. 2013, 441, 701–711.
- Qi, X.; Li, N.; Gu, H.; Xu, Y.; Xu, Y.; Jiao, Y.; Xu, Q.; Li, H.; Lu, J. Amphiphilic oligomer-based micelles as cisplatin nanocarriers for cancer therapy. Nanoscale 2013, 5, 8925–8929.
- Das, M.; Singh, R.P.; Datir, S.R.; Jain, S. Intranuclear drug delivery and effective in vivo cancer therapy via estradiol-peg-appended multiwalled carbon nanotubes. Mol. Pharm. 2013, 10, 3404–3416.
- Arzuman, L.; Beale, P.; Chan, C.; Yu, J.Q.; Huq, F. Synergism From Combinations of Tris(benzimidazole) monochloroplatinum(II) Chloride With Capsaicin, Quercetin, Curcumin and Cisplatin in Human Ovarian Cancer Cell Lines. Anticancer Res. 2014, 34, 5453–5464.
- Huq, F.; Yu, J.Q.; Beale, P.; Chan, C.; Arzuman, L.; Nessa, M.U.; Mazumder, M.E.H. Combinations of Platinums and Selected Phytochemicals as a Means of Overcoming Resistance in Ovarian Cancer. Anticancer Res. 2014, 34, 541–545.
- Nune, M.; Kumaraswamy, P.; Maheswari Krishnan, U.; Sethuraman, S. Self-Assembling Peptide Nanofibrous Scaffolds for Tissue Engineering: Novel Approaches and Strategies for Effective Functional Regeneration. Curr. Protein Pept. Sci. 2013, 14, 70–84.
- Hu, Y.; Wang, H.; Wang, J.; Wang, S.; Liao, W.; Yang, Y.; Zhang, Y.; Kong, D.; Yang, Z. Supramolecular hydrogels inspired by collagen for tissue engineering. Org. Biomol. Chem. 2010, 8, 3267–3271.
- Wang, H.; Wei, J.; Yang, C.; Zhao, H.; Li, D.; Yin, Z.; Yang, Z. The inhibition of tumor growth and metastasis by self-assembled nanofibers of taxol. Biomaterials 2012, 33, 5848–5853.
- Zhang, P.; Cheetham, A.G.; Lin, Y.A.; Cui, H. Self-assembled tat nanofibers as effective drug carrier and transporter. ACS Nano 2013, 7, 5965–5977.
- Soukasene, S.; Toft, D.J.; Moyer, T.J.; Lu, H.; Lee, H.K.; Standley, S.M.; Cryns, V.L.; Stupp, S.I. Antitumor activity of peptide amphiphile nanofiber-encapsulated camptothecin. ACS Nano 2011, 5, 9113–9121.
- Cho, H.; Indig, G.L.; Weichert, J.; Shin, H.C.; Kwon, G.S. In vivo cancer imaging by poly(ethylene glycol)-b-poly(ε-caprolactone) micelles containing a near-infrared probe. Nanomedi. Nanotechnol. Biol. Med. 2012, 8, 228–236.
- Wagh, A.; Singh, J.; Qian, S.; Law, B. A short circulating peptide nanofiber as a carrier for tumoral delivery. Nanomedi. Nanotechnol. Biol. Med. 2013, 9, 449–457.
- Yadav, B.; Taurin, S.; Rosengren, R.J.; Schumacher, M.; Diederich, M.; Somers-Edgar, T.J.; Larsen, L. Synthesis and cytotoxic potential of heterocyclic cyclohexanone analogues of curcumin. Bioorg. Med. Chem. 2010, 18, 6701–6707.
- Sun, A.; Shoji, M.; Lu, Y.J.; Liotta, D.C.; Snyder, J.P. Synthesis of EF24-tripeptide chloromethyl ketone: A novel curcumin-related anticancer drug delivery system. J. Med. Chem. 2006, 49, 3153–3158.
- Somers-Edgar, T.J.; Taurin, S.; Larsen, L.; Chandramouli, A.; Nelson, M.A.; Rosengren, R.J. Mechanisms for the activity of heterocyclic cyclohexanone curcumin derivatives in estrogen receptor negative human breast cancer cell lines. Invest. New Drugs 2011, 29, 87–97.
- Robinson, T.P.; Ehlers, T.; Hubbard IV, R.B.; Bai, X.; Arbiser, J.L.; Goldsmith, D.J.; Bowen, J.P. Design, synthesis, and biological evaluation of angiogenesis inhibitors: Aromatic enone and dienone analogues of curcumin. Bioorganic Med. Chem. Lett. 2003, 13, 115–117.
- Ohori, H.; Yamakoshi, H.; Tomizawa, M.; Shibuya, M.; Kakudo, Y.; Takahashi, A.; Takahashi, S.; Kato, S.; Suzuki, T.; Ishioka, C.; et al. Synthesis and biolgical analysis of new curcumin analogues bearing an enhanced potential for the medicinal treatment of cancer. Mol. Cancer Ther. 2006, 5, 2563–2571.
- Subramaniam, D.; May, R.; Sureban, S.M.; Lee, K.B.; George, R.; Kuppusamy, P.; Ramanujam, R.P.; Hideg, K.; Dieckgraefe, B.K.; Houchen, C.W.; et al. Diphenyl difluoroketone: A curcumin derivative with potent in vivo anticancer activity. Cancer Res. 2008, 68, 1962–1969.
- Liang, G.; Shao, L.; Wang, Y.; Zhao, C.; Chu, Y.; Xiao, J.; Zhao, Y.; Li, X.; Yang, S. Exploration and synthesis of curcumin analogues with improved structural stability both in vitro and in vivo as cytotoxic agents. Bioorg. Med. Chem. 2009, 17, 2623–2631.
- Karthikeyan, N.S.; Sathiyanarayanan, K.I.; Aravindan, P.G.; Giridharan, P. Synthesis, crystal structure, and anticancer properties of cyclic monocarbonyl analogs of curcumin. Med. Chem. Res. 2011, 20, 81–87.
- Dayton, A.; Selvendiran, K.; Kuppusamy, M.L.; Rivera, B.K.; Meduru, S.; Kálai, T.; Hideg, K.; Kuppusamy, P. Cellular uptake, retention and bioabsorption of HO-3867, a fluorinated curcumin analog with potential antitumor properties. Cancer Biol. Ther. 2010, 10, 1027–1032.
- Lee, H.E.; Choi, E.S.; Jung, J.Y.; You, M.J.; Kim, L.H.; Cho, S.D. Inhibition of specificity protein 1 by dibenzylideneacetone, a curcumin analogue, induces apoptosis in mucoepidermoid carcinomas and tumor xenografts through Bim and truncated Bid. Oral Oncol. 2014, 50, 189–195.
- Novaković, M.; Pešić, M.; Trifunović, S.; Vučković, I.; Todorović, N.; Podolski-Renić, A.; Dinić, J.; Stojković, S.; Tešević, V.; Vajs, V.; et al. Diarylheptanoids from the bark of black alder inhibit the growth of sensitive and multi-drug resistant non-small cell lung carcinoma cells. Phytochemistry 2014, 97, 46–54.
- Zheng, A.; Li, H.; Wang, X.; Feng, Z.; Xu, J.; Cao, K.; Zhou, B.; Wu, J.; Liu, J. Anticancer Effect of a Curcumin Derivative B63: ROS Production and Mitochondrial Dysfunction. Curr. Cancer Drug Targets 2014, 14, 156–166.
- Ucisik, M.H.; Küpcü, S.; Schuster, B.; Sleytr, U.B. Characterization of CurcuEmulsomes: Nanoformulation for enhanced solubility and delivery of curcumin. J. Nanobiotechnol. 2013, 11, 37.
- Sahu, A.; Kasoju, N.; Goswami, P.; Bora, U. Encapsulation of curcumin in Pluronic block copolymer micelles for drug delivery applications. J. Biomater. Appl. 2011, 25, 619–639.
- Basniwal, R.K.; Khosla, R.; Jain, N. Improving the anticancer activity of curcumin using nanocurcumin dispersion in water. Nutr. Cancer 2014, 66, 1015–1022.
- Esmaili, M.; Ghaffari, S.M.; Moosavi-Movahedi, Z.; Atri, M.S.; Sharifizadeh, A.; Farhadi, M.; Yousefi, R.; Chobert, J.M.; Haertlé, T.; Moosavi-Movahedi, A.A. Beta casein-micelle as a nano vehicle for solubility enhancement of curcumin; food industry application. LWT-Food Sci. Technol. 2011, 44, 2166–2172.
- Anuchapreeda, S.; Fukumori, Y.; Okonogi, S.; Ichikawa, H. Preparation of Lipid Nanoemulsions Incorporating Curcumin for Cancer Therapy. J. Nanotechnol. 2012, 2012, 270383.
