Monoclonal Abs detect only one epitope, because they are produced from a family of genetically stable cells (cloned hybridoma); consequently, they have high specificity to the Ag than the polyclonal Abs that are less specific, because they are produced by different cell clones.
- drugs of abuse
- lateral flow assay (LFA)
- immunochromatography
- monoclonal antibodies and detection
1. Introduction
Monitoring the use of psychotropic drugs is a public health issue and is proving to be very useful in the workplace. This is because illicit drugs can affect the cognitive and motor functions [1][2][3][4][5][6][7][8][9][1,2,3,4,5,6,7,8,9]. In addition, many psychoactive drugs, especially benzodiazepines (BZDs), are strongly associated with illicit drug use [10].
The concentrations of psychotropic drugs in biological matrices (saliva, blood, urine, etc.) are low, which requires very sensitive, selective, and appropriate methods for their detection and quantification [11][12][13][14][15][16][17][11,12,13,14,15,16,17]. The most frequently used methods of drug detection today are based on procedures for separating compounds by chromatographic standard techniques (GC, HPLC, and LC). Their quantifications are then carried out using UV, electrochemical, or fluorescence detectors. The GC-MS or, even more, LC-MS, are the most efficient methods of analysis [11][16][18][19][11,16,18,19]. However, they are expensive and require trained and qualified clinicians to conduct the analyses. Every year—in order to simplify detection methods and reduce costs—new lateral flow immunoassay (LFIA) tests appear in all fields of medicine, including cancerology, toxicology, and infectology [17][18][19][20][21][22][23][24][25][26][17,18,19,20,21,22,23,24,25,26], to be used directly in places where patients are cared for, with no specialized laboratories. The goal is to have immediate results, so that certain diagnoses can be quickly included or excluded.
The LFIA tests have several advantages over conventional diagnostic tests. They provide immediate treatments in the events of potentially life-threatening diseases, specific treatments rather than presumptive treatments, and early measures to prevent transmission of the disease (i.e., in the hospital or the community). In addition, these tests avoid unnecessary treatments and further investigation through follow-up testing [27]. Despite these advantages, each LFIA has its own characteristics, which must be known by the practitioners if they want to use it correctly.
These assays are based on the antigen (Ag)-antibody biochemical interaction and their performances depend essentially on the characteristics of the antibodies (Abs), such as affinity, specificity, production process (i.e., monoclonal Ab or polyclonal Ab), and cross-reactivity [28]. Abs are the primary reagent used for LFIA for the detection of low concentrations of analytes (the drug consumed) in the sample. Their selection (Abs) is a critical step of LFIA development. For a competitive LFIA, which is most useful in the case of small molecules, such as drugs of abuse (DOA), we used only one antibody (Ab) that was sensitive and specific to the target molecule. In the case of a sandwich LFIA, we used two Abs that could bind to the analyte with high specificity and sensitivity, but before using them, we had to test the available pairs, to determine which pair met the requirements. In general the Abs most used by the majority of the authors are monoclonal antibodies (mAbs), in which is fixed a compound (gold nanoparticles, latex microbeads, etc.) that will allow the visualization of the Ag-Abs reaction [27]. Monoclonal Abs detect only one epitope, because they are produced from a family of genetically stable cells (cloned hybridoma); consequently, they have high specificity to the Ag than the polyclonal Abs that are less specific, because they are produced by different cell clones [28].
2. Antibodies Production Processes: Focus on mAbs against Drugs of Abuse
The Abs are glycoproteins called immunoglobulins (Ig), secreted by B-lymphocytes, components of the adaptive immune system, in response to an immunogen. There are many different isotypes or classes (IgG, IgA, IgM, IgE, and IgD), but the IgG isotype is often the major component of commercially available Abs and constitutes the most fractions of blood proteins. IgG is further divided into four subclasses (IgG1, IgG2, IgG3, and IgG4), with the numbers corresponding to the decreasing order in which they are found in the blood [29][30].
Abs production is simple, but there are several factors that affect the probability of an animal to produce Abs against the injected Ag (immunogen). The factors influencing immunogenicity are [30][31]:
-
The molecular size of the injected Ags: the most active immunogens tend to have a high molecular mass (>14,000 Da). Indeed, small Ags (e.g., DOA) are known to be either non-antigenic or weakly antigenic.
-
The foreignness: an antigen must be a foreign substance to the animal (not self) to elicit an immune response.
-
The chemical complexity: the more complex the immunogen or substance is (chemically), the more immunogenic it will be. The DOA (BZD, heroin, amphetamine, morphine, etc.) are often of low molecular weight and, generally, for any very small Ag, the entire chemical structure is considered by the immune system as a single epitope to which an Ab binds.
Since they are unable to induce an immune response by themselves, they require a carrier molecule to act as a complete Ag. They are used as haptens or as a recognition site for the production of specific Abs by coupling them to a suitable carrier molecule (the immune response in the host animal can produce Abs against the entire immunogen and not just the drug molecule). Many proteins can be used as carriers, but the most commonly used ones are bovine serum albumin (BSA; 67 kDa) and keyhole limpet hemocyanin (KLH, 400 kDa), which are highly immunogenic because of their complexity (structure) and large sizes [31][32][33][34][32,33,34,35].
BSA is widely used as a blocking agent in development of immunoassays, such as ELISA and LFIA, because it is very accessible and available and has numerous useful groups to be linked to small molecules, including DOA, as a carrier molecule to induce the immune system. For this reason, it is recommended, for example, to use KLH as the carrier molecule (protein) to induce an immune response against the hapten and the BSA for Abs screening and purification, to assure the detection of the Ag (hapten) instead of the carrier Abs [31][32][34][32,33,35].
If morphine (MOP) is taken as a model for developing a DOA detection system based on LFIA, the carbon atoms in its positions 3, 6, 2, and group N, readily lend themselves to conjugation to the carrier protein (Figure 1).
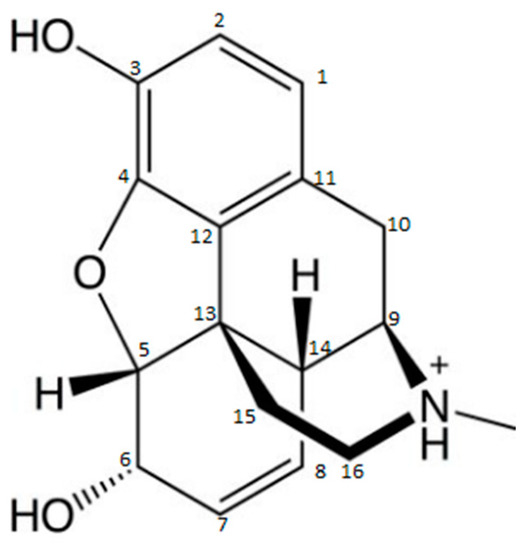
Figure 1. Molecular structure of morphine [35].
The production of Abs directed against the MOP molecule using a carrier molecule in group N to produce a more specific assay for MOP detection is commonly used. This leaves positions 3 and 6 as antigenic determinants and, thus, allows the production of Abs more likely to be specific to MOP, without cross-reactivity to codeine (COD) or dihydrocodeine, for example. However, if the immunogen is produced via position 3, it is generally used to produce broad cross-reactive anti-opiate Abs (diacetylmorphine and MOP-3-glucuronide). Cross-reactivity to different opiates varies from one Ab to another. It is important that each Ab is fully characterized by the test developer. However, the production of MOP Abs in position 6 gives a better specificity to MOP relative to COD and MOP-3-glucuronide, and is expected to produce Abs with good cross-reactivity with 6-monoacetylmorphine and the active metabolite MOP-6-glucuronide [36][37].
When a mammal animal, such as a mouse, rabbit, sheep, goat, rat, or a horse (for large quantities of Ab) is immunized with an Ag immunogen, it will cause stimulation of all B-lymphocytes that produce Abs specific to that Ag. This stimulation will result in the clonal multiplication of these B-lymphocytes, which will turn into plasmacytes secreting the specific Ig. A clone produces the same Ig that has the same specificity for a given epitope. The Abs derived from a clone of plasmacytes are called monoclonal Abs (same Abs produced from a single clone).
The majority of the mAbs available in the market are IgG isotype because of their superior affinity and specificity compared to the other isotypes (IgM). However, in the natural situation, an Ag always produces a polyclonal serum [37][38]. Thus, the combination of Igs derived from different clones, but all recognizing different epitopes of the same Ag forms a polyclonal serum, called antiserum, specific for the given Ag.
The measure of binding strength between an Ag and an Ab is described by the affinity constant. This binding is non-covalent, reversible, and reaches equilibrium. In addition, high affinity Abs bind faster than low affinity ones and perform better in immunochemical methods [37][38].
In general, the commercial production of recombinant monoclonal antibodies (mAbs) follows principally similar workflow. The process begins with the generation of a mAb by immunizing an animal or by techniques using molecular biology methods involving the identification and optimization of the genetic coding sequence and the construction and identification of a stable high-producing clone.
Today, in the laboratory step, several techniques are well established and commonly used to obtain mAbs, namely: Epstein-Barr virus (EBV) lymphoblastoid transformation technique, hybridoma technique, and phage display technique (Scheme 1) [38][39].
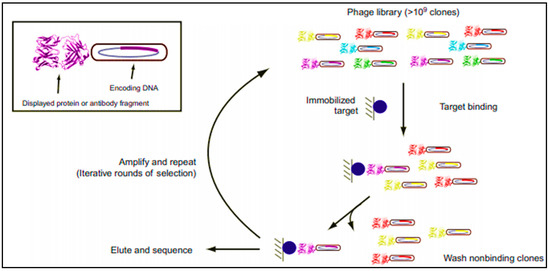
Scheme 1. Schematic representation of phage display process. It consists of cycles, each including: incubation of the antibody (Ab) repertoire and the target, washing of nonspecific binders, elution, and amplification of specific binders for further cycle or for screening [39].
Phage display technique is the most commonly applied technology to produce recombinant antibodies in the laboratory settings. This helps the isolation of proteins from diverse mutagenic libraries and investigates protein-protein, protein-peptide, and protein-DNA interactions, and consists, basically, in cloning Fab coding genes into bacteriophage plasmid vectors [38][39]. The advantages of this methodology are multiple: one library can generate a great number of new Abs, it is an in vitro process (so animal immunizations steps are not required), and, accordingly, even toxic antigens can be tested. Moreover, the Abs molecules can be rapidly obtained [38][39][40][41][39,40,41,42]. However, for LFIA development application, mAbs produced using this technique are still not widely used, and the mostly used ones are derived from mouse hybridoma (but exhibit a downside in human therapeutics).
In 1975, Georges Köhler and Cesar Milstein described the first technique developed for stable monoclonal antibody production. This technique consists of creating a hybridoma, a stable hybrid cell capable of producing a single type of antibody against a specific epitope present in an antigen (Scheme 2). It is also called the technique of hybridization cell and is a method for producing large numbers of mAbs. In LFIA development application (immunoassay diagnostic or screening tests in general), it is the mostly used technique to produce mAbs in all laboratories, but has a downside in human therapeutics. The hybridoma technique is currently performed following four main steps (Scheme 2):
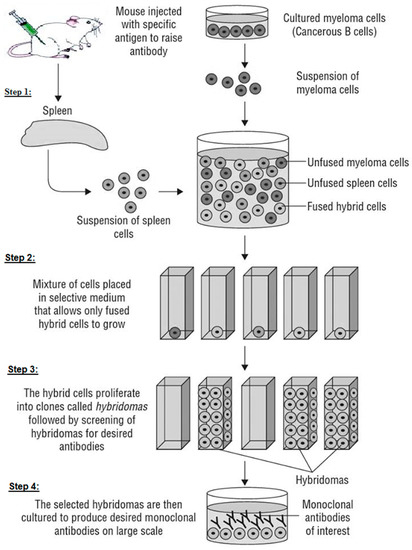
Scheme 2.
-
Step 1: fusing the secretory lymphocyte of an Ab to the Ag used in the animal’s immunization with the myeloma using polyethylene glycol.
-
Step 2: identifying the Ab secretory hybridoma.
-
Step 3: isolating one cell and maintaining it in culture to obtain a single clone or family of cells, all of which are identical and secretive of the same mAb. It is limit-dilution cloning, and several successive clones are sometimes necessary to obtain a genetically stable clone.
-
Step 4: growing the cloned hybridoma in a bioreactor to obtain a mAb concentrate or in a roller system to obtain the less concentrated mAb as a culture supernatant. It can be injected into the abdomen of BALB/c mice (Bagg albino, laboratory-bred strain of the house mouse) to obtain ascites-concentrated mAb.
Overall, an immunization program usually involves injecting three to six animals with the same Ag. However, if appropriate Abs are not produced after multiple immunizations, it may be necessary to repeat the program with different animals and possibly a different immunogen [36][37].
This method was used by several authors for the development of mAbs against DOA. Indeed, Dehghannezhad et al. in 2012 [42][43] used it to produce a mAb and conjugated it to gold nanoparticles (GNPs) to develop a rapid competitive immunochromatographic strip test to detect MOP in urine samples. It was also used to develop Abs for the diagnosis and screening of different diseases and clinical cases, including arthritis, breast cancer, psoriasis, leukemia, transplant rejection, asthma, and toxicity [43][44][45][46][47][48][49][50][51][44,45,46,47,48,49,50,51,52].
3. Performances of an Antibody
After the Ab is produced, as described before, surface plasmon resonance (SPR), equilibrium dialysis, ELISA, or many other methods are widely used to indicate its affinity (termed as binding strength or binding constant) to the Ag, and demonstrate its characteristics and binding to the target drug in real-time, and in a label-free manner, using a refractive index change at a metal surface [52][53][53,54]. There is also the possibility of using ELISA to verify that the Ab meets the need with the target drug (i.e., sensitivity, specificity). In this way, the binding and displacement can be observed with each Ab. Careful titration of the Abs and labeled drug derivative may improve the assay characteristics, and then the assay may be further optimized by the addition of other proteins, surfactants, and stabilizers to the assay buffer.
3.1. Applications of mAbs and Their Comparison with Polycolonal Antibodies in the Development of LFIA
Specific mAbs provide accurate testing. They are used for the determination of ABO and rhesus blood groups, for HLA tissue grouping, for the immunolabeling of acute leukemia and for the development of immunological tests (enzyme-linked immunosorbent assay (ELISA), lateral flow immunoassay (LFIA), radioimmunoassay (RIA), etc.) [18][19][20][21][27][30][54][55][56][57][58][59][18,19,20,21,27,31,55,56,57,58,59,60]. Other Abs issued from animals conjugated to markers or enzymes are used for diagnostic kits manufacturing, immunocytochemical analysis, and research [18][19][20][21][27][30][54][55][56][57][59][60][61][18,19,20,21,27,31,55,56,57,58,60,61,62].
Their frequent usage in basic research has led to the study and the understanding of many biological processes. Moreover, a panel of mAbs is usually used to map and study the role of epitopes in certain cellular functions and mechanisms. They have also an important role in proteomics and mass biological screening tests [18][19][20][21][30][42][54][55][57][58][18,19,20,21,31,43,55,56,58,59]. In general, mAbs are used in diagnostic, agri-food, veterinary, microbiological, and toxicological tests [20][21][30][55][57][62][20,21,31,56,58,63]. However, mAbs generally have less affinity than pAbs, which may lead to less sensitive assays. It should be noted that in drug detection tests, an Ab may be too specific as it may be desirable to have broad cross-reactivity with a family of drugs (such as BZDs) or with a single drug and its metabolites (such as buprenorphine).
The mAbs offer the advantages of purity and homogeneity, which is useful in the circumstances where the Ab is labeled or conjugated within the framework of the LFIA’s development [60][63][64][61,64,65]. They all recognize specifically a single epitope and are homogeneous compared to pAbs, which allows the testing to be standardized. The monospecificity provided by mAbs, makes it possible to understand and evaluating changes in molecular conformation and structure, phosphorylation states, protein-protein interactions, and in identifying single members of protein families. However, the monospecificity of mAbs may also limit their advantages, because they should be generated to the Ag epitope to which it will bind (small change in the structure of an epitope can affect the function of a mAb) [37][65][38,66]. They can also identify an antigenic determinant in complex mixtures, such as biological fluids (blood, urine, milk, saliva, etc.).
Both polyclonal and monoclonal antibodies have their own advantages and disadvantages, which make them useful for different applications. The debate regarding whether mAbs are better than pAbs has been raging for years. Some researchers praise the batch-to-batch consistency and single-isotype nature of monoclonals, others swear by the ability of polyclonals to work in a wider range of applications, often enabling detection of the target antigen in both its native and denatured states.
The pAbs are heterogeneous and have a wide specificity than mAbs, because they are produced by a large number of B cell clones, each generating pAbs to a specific epitope. The pAbs’ production techniques are easy, fast, and low-cost compared to mAbs’ production techniques. However, their production costs depend on the quantities required and their use.
The best use of pAbs is to detect unknown antigens. pAbs are used as a secondary antibody (detectors) in immunoassays (e.g., ELISA, western blotting, microarray assays, immunohistochemistry, flow cytometry). Their role is to bind to different epitopes and amplify the signal, leading to better detection. When pAbs are used as detectors, more steps are needed, such as labeling and affinity purification, which may increases the costs. Moreover, a production of large quantities of PAbs requires a large number of animals, restrictive farming conditions, and expensive infrastructure.
In contrast, mAbs are often used as primary antibodies in immunoassays because of their ability to bind specifically to a single epitope of an Ag. They are easy to label and provide an unlimited source of antibody that is homogeneous and, once characterized, predictable in its behavior. Nowadays, mAbs specificity can be expanded by combining multiples mAbs that lead to the capture of multiple epitopes of an Ag.
Another advantage of mAbs is that, once their line is established, their supply is infinite, and the risk of isolating the desired cell line never has to occur again. In the opposite way, pAbs are prone to batch-to-batch variability and there is no guarantee that immunizing other animals will yield to a useable Ab.
