Eukaryotic cells, are complex cells that evolved through endosymbiosis when one cell (typically bacterial, forming mitochondria and plastids) is incorporated by a host cell. It might well be that other cellular organelles are also of endosymbiotic nature.
- actin
- cell
- cell biology
- cytoskeleton
- consciousness
- eukaryotes
- excitability
- membranes
- sentience
- symbiosis
1. Chimeric Nature of the Eukaryotic Cell
In addition to prokaryotic bacteria and archaea, eukaryotic protozoa are considered to represent unicellular organisms. However, protozoa, as well as all other eukaryotic cells, are complex cells that evolved through endosymbiosis when one cell (typically bacterial, forming mitochondria and plastids) is incorporated by a host cell. It might well be that other cellular organelles are also of endosymbiotic nature. The difficulty is that over geological time, large amounts of DNA can be lost, as in the case of the highly reduced nuclei known as nucleomorphs, in which almost all of the DNA is transferred to the host cell nuclei [1][2]. Similar processes reduced the genome complexity of plastids and mitochondria during their endosymbiont-to-organelle transition for plastids see [3]. Recently, we [4][5][6] discussed the endosymbiotic origin of the eukaryotic nucleus that occurred when a host cell enclosed and endogenized a guest cell of apparent archaeal origin. In this proposal, all the host cell DNA is transferred to the guest cell, which is transformed into the eukaryotic nucleus [6]. In fact, one cannot exclude a putative endosymbiotic origin of several other organelles, such as endoplasmic reticulum, peroxisomes, centrosomes/centrioles and cilia/flagella [7][8][9][10]. In cellular evolution, cell–cell merging and endosymbiosis is an ancient and successful strategy, representing a fundamental feature and can also be seen in instances of the secondary and tertiary endosymbiotic events in algae [1][2][3][11][12]. In endosymbiosis, tinkering rather than whole-scale re-engineering is obvious [13] when large structures are continuously rearranged and recombined after cellular mergings of the formerly independent unicellular organisms [14][15].
23. Structures and Processes Behind Cellular Consciousness—Evolution of Chimeric Consciousness of Eukaryotic Cell
Lynn Margulis was one of the first scientists to seriously discuss the evolutionary origin of cellular consciousness and argued that prokaryotic cells that merged to form chimeric eukaryotic cells had their own prokaryotic-specific sentience [8]. In her view, the original prokaryotic cells had a “protoconsciousess”, and the two merged cells generated a supracellular consciousness. We develop this below from the perspective of the actin- and tubulin-based cytoskeletal elements where the host cell is proposed as a large archaea cell based on the actin cytoskeleton, while the small motile guest cell is based on the tubulin cytoskeleton supported by the centrosome and basal bodies/centrioles that animate eukaryotic flagella [16][4][5][6].
We recently discussed the biological foundations of cellular consciousness based on how an excitable plasma membrane, densely populated with so-called biological Maxwell demons, such as sensors, receptors, ion channels, transporters, and ATPases, can generate a senomic cellular field [17][18][19][20][21]. In the evolutionary origins of the eukaryotic cell (
Box 1), both the large, actin-based host cell and the smaller guest cell, which relied on the tubulin-based cytoskeleton, were proposed to be ancient archaea [4][5][6]. This may allow the merging of their fields to generate the new stronger and senomic field of an emergent eukaryotic cell. In addition to the excitable plasma membrane and membranes of recycling vesicles, other cellular structures that are capable of contributing to the cellular fields are the large, bundled, vibrating elements of the cytoskeleton (
Box 2), such as F-actin [22][23][24][25] and microtubules [26][27][28][29]. Both excitable plasma membrane and cytoskeletal elements have been proposed to generate proto-consciousness of individual eukaryotic cells [17][30].
Box 1.

Box 2.

Vibrations of excitable polymers contribute to the intracellular electromagnetic fields and can be expected to interact with the field emanating from the excitable plasma membrane. As microtubules act as memristors, as combinations of memory and electromagnetic resistance [31][49], they are well suited to faithfully decode the cellular senomic fields and to act accordingly. Furthermore, microtubules are structurally linked to both the actin filaments as well as the plasma membrane; they are perfectly suited to generate subcellular bioelectric circuits [31][32][49,50].
34. Structures and Processes behind Cellular Consciousness—Two Types of Nanobrains Generating Consciousness of Eukaryotic Cell
Two ancient cells merging into one resulted in the generation of supracellular chimeric consciousness having four different excitable sources: two plasma membranes, F-actin, and microtubules. The plasma membrane of the host cells, associated with the actin cytoskeleton, produced the senomic fields of contemporary chimeric eukaryotic cells. The guest cell transformed into the eukaryotic nucleus with the centrosome associated with centriole and organizing perinuclear microtubules [33][51]. The plasma membrane and the nuclear envelope/centrosome/microtubules complex can be viewed as two different cellular nanobrains, the origin, of which can be traced back to the two ancient cells, which merged together, forming the first eukaryotic cell [4][5][6][15,16,17]. Vibrations of F-actin and microtubules contribute significantly to the cellular electromagnetic field [27][28][45,46]. As microtubules act both as intracellular nanowires and memristors, they are perfectly suited for the nanobrain roles of the centrosomes/nuclear envelopes, complementing the principle nanobrain of the eukaryotic cell represented by the excitable plasma membrane (Figure 1, Box 1 and Box 2) inherently linked to the actin cytoskeleton.
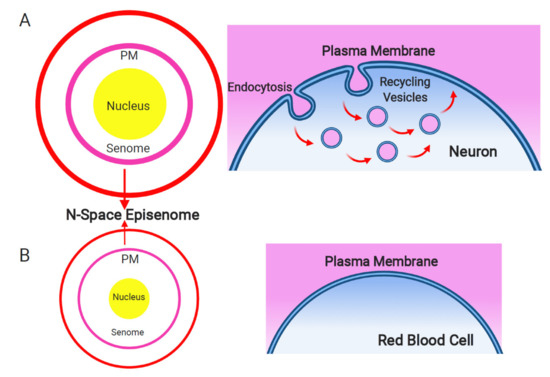
Figure 1. Plasma membrane and endosomal recycling vesicles-based nanobrain. Schematic depiction of the senome and the N-space episenome in two contrasting cells of multicellular organisms. (A) In the neurons and neuron-like cells, highly active endocytosis and endocytic vesicle recycling results in hypertrophied senome (lilac circle) and N-space episenome (red circle). Such cells are well-informed about their environment and are active in cell–cell communication via their plasma membrane-based nanobrains. (B) In the example of mature red blood cells, there are only minimal activities of endocytosis and endosomal vesicle recycling. Such cells have shrunk their senomes (lilac circle) and N-space episenomes (red circle) based nanobrains. They are socially isolated, with minimal cell–cell communication and highly reduced cellular sensory apparatus.
