Vaccines constitute the most effective medications in public health as they control and prevent the spread of infectious diseases and reduce mortality. Similar to other medications, allergic reactions can occur during vaccination. While most reactions are neither frequent nor serious, anaphylactic reactions are potentially life-threatening allergic reactions that are encountered rarely, but can cause serious complications. The allergic responses caused by vaccines can stem from activation of mast cells via Fcε receptor-1 type I reaction, mediated by the interaction between immunoglobulin E (IgE) antibodies against a particular vaccine, and occur within minutes or up to four hours.
- allergy
- anaphylaxis
- COVID-19
- Kounis syndrome
- vaccines
1. Introduction
Vaccines constitute the most effective medications in public health as they control and prevent the spread of infectious diseases. From the mid of the 20th century, many infectious diseases and their sequels have been effectively reduced, eliminated, and avoided through the use of vaccinations [1].
However, similar to other medications, allergic reactions can occur during vaccination. Vaccine-associated allergic reactions are neither frequent nor serious. However, anaphylactic reactions are potentially life-threatening, and can occur immediately, usually within minutes, after exposure to a vaccine. Such reactions are encountered rarely, but can cause serious complications. Following an allergic reaction after vaccination, it is difficult to discriminate and ascertain whether the reaction was caused by the vaccine itself or by other factors. The increased incidence of viral epidemics has rendered an increased use of various vaccines, and therefore, mild allergic reactions are frequently encountered in everyday clinical practice. However, even such mild allergic reactions can still lead to serious complications, and therefore, prompt attention, prevention, and treatment are required [2]. In the United States, the Advisory Committee on Immunization Practices (ACIP) recommends an immunization schedule in children to receive a total of 10 vaccines to protect against 16 diseases before the age of two years [3]. According to a recent study, the risk of an allergic reaction post-vaccination is estimated to be 1.31 (95% CI, 0.90–1.84) per million vaccine doses [4].
2. Pathophysiology of Vaccine-Induced Allergic Reactions
The allergic reactions caused by vaccines can stem from the following pathophysiologic mechanisms (Figure 1):
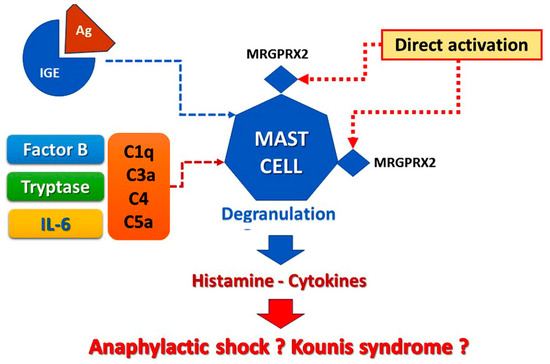
Figure 1. Vaccine-induced allergic reaction.
a. Reactions via the pathway of mast cell activation and degranulation as IgE/antigen through cross-linking of FcεRI on mast cells [5][6]. This activation of mast cells via Fcε receptor-1 constitutes the hallmark of classical Gell and Coombs Type I of hypersensitivity reactions [5][6]. This type of reaction is the most important and well-understood. Especially, type I reactions are mediated by the interaction between IgE antibodies against a particular vaccine component acting as antigen and usually occur within minutes or up to 4 h of exposure to the relevant antigen [2]. This mechanism is confirmed by the specific IgEs detection and the increased levels of serum tryptase [6][7]. The contained excipients are considered as the most probable cause of IgE-mediated allergic reactions, whereas the vaccine antigen and the residual non human protein should always be regarded as the less probable cause of the allergy, referring to the currently approved COVID-19 vaccine [7][8].
b. Non-IgE-mediated mast cell degranulation is performed via activation of the complement system that leads to the generation of anaphylatoxins C1q, C3a C4, and C5a and Factor B, which are strong inflammatory stimulators able to induce mast cell activation and degranulation. This complement pathway activation and positive bio-feedback loops involving interleukin-5 (IL-5) and tryptase are much more common than recognized involving patients who develop renal failure or fatal cerebral events [6][8][7,9].
c. Life-threatening allergic reactions can be mediated via direct activation of the Mas-related G protein-coupled receptor X2 (MRGPRX2). MRGPRX2 factors that may activate mast cells via non-Fcε receptors are clinically and significantly enhancing our ability to prevent those reactions via MRGPRX2 antagonists’ identification that could further serve as new therapeutic strategies [9][10]. Notably, in MRGPRX2 activation of mast cells, the specific IgEs may remain undetected, and tryptase levels may be normal even in so serious Kounis syndrome [7][8].
d. Type IV hypersensitivity or delayed reactions generally initiate 48 h after vaccination demonstrating their peak between 72 and 96 h [2]. They constitute the second most common type of hypersensitivity reactions, are cell-mediated and antibody independent, are derived from overstimulation of T cells and monocytes/macrophages and release of cytokines that cause inflammation, cell death, and tissue damage. Vaccines containing anti-microbial agents and ingredients, such as thimerosal and aluminum, can be followed by delayed reactions [2]. For further understanding of the reactions caused by the current COVID-19 vaccination, it is important to consider all the above mechanisms and pathways.
3. Causality of Vaccine Allergy
Allergic reactions to vaccines are rarely attributed to the active vaccine itself, as they might be caused to inactive ingredients, such as egg protein, gelatin, formaldehyde, thimerosal, or neomycin, that contribute to specific IgE-mediated immediate reactions (Figure 2). Excipients, according to European Medicines Agency (EMA), are constituents of a pharmaceutical form apart from the active substance [10][11]. Excipients constitute inert substances that are added to vaccines and other drugs in an effort to improve stability, increase solubility, improve absorption, influence palatability, or create a distinctive appearance. Excipients can cause a variety of allergic clinical manifestations ranging from skin disorders to life-threatening systemic reactions. In particular, necessary excipients added to vaccines to stimulate a stronger immune response, stabilize the potency of the vaccine during transportation and storage, or to prevent contamination by bacteria, can further trigger allergic reactions. Consequently, excipients represent major contributors to the development of specific IgE-mediated and immediate reactions, associated with vaccines [7][8]. Other excipients, like polyethylene glycol (PEG) and polysorbate, used to improve water-solubility in drugs, may also cause allergic reactions [11][12]. The variety of excipients contained in various medications is depicted in Table 1. Polysorbate and PEG excipients are components in many vaccines and in injectable medications [12][13]. Immunoglobulin Μ (IgM) and immunoglobulin G (IgG) can induce complement activation-related pseudo-allergy (CARPA), which constitutes a nonspecific immune response to PEGylated nanoparticle-based medicines [13][14]. This pathway may be responsible for reactions to medications, such as liposomal doxorubicin and other drugs in clinical trials [14][15].
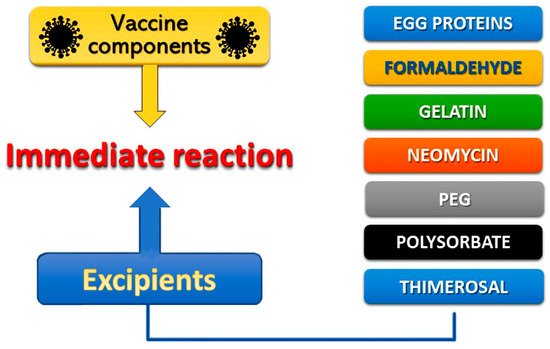
Figure 2. Vaccine compound allergens.
Table 1. Excipients that can induce allergic reactions, contained in various medications shown in parenthesis.
| Ascorbyl tetraisopalmitate |
| (topical medications) |
| Benzalkonium chloride |
| (corticosteroids, ophthalmic medications) |
| Benzyl alcohol |
| (parenteral medications, topical medications) |
| Carboxymethylcellulose |
| (corticosteroids, wound dressings) |
| Cetostearyl alcohol |
| (topical medications) |
| Chlorocresol |
| (topical medications) |
| Colloidal silica |
| (NSAID) |
| Colophonium |
| (wound dressings) |
| Dioctyl sodium sulfosuccinate |
| (topical medications) |
| Ethylenediaminetetraacetic acid (EDTA) |
| (topical medications) |
| Formaldehyde |
| ( |
| VACCINES |
| ) |
| 1,2,6-Hexanetriol |
| (topical medications) |
| Imidazolidinyl urea |
| (ultrasound gels) |
| Isopropyl palmitate |
| (topical medications) |
| Metacresol |
| (insulin) |
| Methyldibromo glutaronitrile |
| (ultrasound gels) |
| Parabens |
| (topical medications) |
| Polyethylene glycol |
| ( |
| VACCINES |
| , foods, lubricants, medications, skin creams) |
| Polysorbate |
| ( |
| VACCINES |
| , anticancer agents, creams, lotions, medications, ointments, vitamin oils) |
| Propyl gallate |
| (topical medications) |
| Propylene glycol |
| (antihistamines, anxiolytics, lubricants, topical medications, ultrasound gels) |
| Sodium metabisulfite |
| (local anesthetics, topical medications) |
| Sodium sulfite |
| (topical medications) |
| Sorbitansesquioleate |
| (topical medications) |
| Sunset Yellow |
| (mineral supplements) |
| Thimerosal |
| (ophthalmic medications) |
On the other hand, allergic reactions to the viral or microbial component itself have been implicated rarely in atopic patients after vaccination with Bordetella pertussis antigens, tetanus, and diphtheria toxoids, or pneumococcus vaccine [15][16]. Delayed urticaria, angioedema, and nonspecific skin rashes have been reported frequently (5% to 13%) in patients receiving such vaccines [16][17]. In the above reports, however, detailed and meticulous allergic testing to confirm an immune-mediated allergic reaction was not performed.
