Diabetic retinopathy (DR) is the most frequent microvascular complication of long-term diabetes and the most common cause of blindness, increasing morbidity in the working-age population. The most effective therapies for these complications include laser photocoagulation and anti-vascular endothelial growth factor (VEGF) intravitreal injections. However, laser and anti-VEGF drugs are untenable as a final solution as they fail to address the underlying neurovascular degeneration and ischemia. Regenerative medicine may be a more promising approach, aimed at the repair of blood vessels and reversal of retinal ischemia. Stem cell and tissue engineering therapy have introduced a novel way to reverse the underlying ischemia present in microvascular complications in diseases such as diabetes.
- diabetic retinopathy
- ischemia
- stem cell
- vascular regeneration
- vascular tissue engineering
1. Introduction
Diabetes mellitus is a chronic metabolic disease characterized by sustained hyperglycemia that leads to macro and microvascular complications[1] [1]. Diabetes is the leading cause of blindness among adults aged between 20 and 79 years old. Recent surveys have predicted that by 2030, the number of patients with diabetes mellitus will increase to 440 million worldwide (prevalence 7.7%)[1] [1]. Globally, diabetes will lead to an increasing incidence of two major types of late complications: macrovascular and microvascular, which cause greater morbidity and premature death. Cerebrovascular, cardiovascular, and peripheral vascular diseases are examples of macrovascular disorders in which large vessels are affected. In contrast, microvascular complications affect small vessels and include nephropathy, neuropathy, and retinopathy. Retinopathy is one of the most common ischaemic disorders of the retina and the main cause of blindness in the working-age population. It is responsible for 12,000–24,000 new cases of blindness each year worldwide[2][3][4] [2–4].
Diabetic retinopathy (DR) manifests as a broad spectrum, particularly at the level of the retinal vasculature, and is responsible for 4.8% of the 37 million cases of blindness in the world according to the World Health Organization (WHO). The main risk factors for DR are high blood pressure, hyperglycemia, and the duration of diabetes. Studies have found a consensus that there is a pathogenic link between hyperglycemia and the onset and progression of DR, while tight control of blood glucose can delay DR onset and progression. Some of the DR risk factors are gender, age at onset of the disease, ethnicity, cataract extraction, and hyperlipidemia[2] [2]. The duration of diabetes is another main risk factor for DR. Although type 1 and type 2 diabetes have some different phenotypic variations, the prevalence of diabetic retinopathy in both populations after 10 years is approximately 75% which increases to 90%–95% after 20 years. Despite the increasing number of diabetic patients during the last decade, most of therapeutic applications only result in reducing the pathogenic process and not affecting the underlying cause of the DR. Therefore, there is an urgent need to investigate novel approaches to address the problem. In this review, we first explain the pathogenesis of DR and current therapeutic approaches and then will discuss novel cell base and tissue engineering approaches. Tissue engineering strategies have three basic components: first, the cell source which must express the appropriate genes and maintain the appropriate phenotype in order to preserve the specific function of the tissue[5] [5]. Second, the bio-reactive agents or signals that induce cells to function. third, the scaffolds that house the cells and act as a substitute for the damaged tissue[6] [6]. The source may be either embryonic stem cells (ESC) or adult stem cells (ASC), the scaffolds may be categorized as synthetic, biological, or composite, and the signals may include growth factors/cytokines, adhesion factors, and bioreactors[5] [5].
2. Vascular Insufficiency and Inner Retinal Ischemia in Diabetic Retinopathy
Ischemia is characterised by the restriction of blood supply to tissue and organs, causing a shortage of oxygen and glucose which is needed for cellular metabolism and removal of metabolites[3] [3]. Ischemia-related pathologies are central to many diseases and pose a challenge for healthcare systems worldwide. Angina, myocardial infarction, stroke, and ischaemic retinopathies are some of the most common ischemia-related diseases which represent a major cause of morbidity and mortality worldwide[6] [6].
Vaso-degenerative retinopathies, such as DR, can result in variable degrees of retinal vascular insufficiency and a profound loss of vision. Beyond the significant risk of depriving delicate neural networks of oxygen and nutrients, hypoxia also increases growth factor and cytokine expression. This can result in vascular leakage in the surviving vasculature and/or pre-retinal and papillary neovascularization. If these complications are left untreated, the responses to vascular stasis, ischemia or hypoxia can result in fibro-vascular scar formation or retinal edema and blindness[3][7] [3,7].
3. Clinical Signs and Diagnosis
Many diabetic patients may not experience any noticeable symptoms in the early stage of the disease. However, early detection of DR can help to prevent severe loss of vision and blindness. Different clinical signs of retinopathy include dot and blot retinal hemorrhage, the formation of microaneurysms, cotton wool spots, hard exudates, venous abnormalities, and growth of new blood vessels. There are also anatomical changes during DR that have been well-documented and include the formation of acellular capillaries, early thickening of the basement membrane, formation of microaneurysms, loss of pericytes and endothelial cells, and retinal neovascularization[8] [8]. DR diagnosis involves visual acuity testing, fundus examination (direct and indirect ophthalmoscopy), and retinal photography. Optical coherence tomography (OCT) is widely used to examine the major layers of the retina and the various reflectance of visible light[9][10] [9,10]. By using this technique, it is possible to localize retinal lesions in relation to different retinal layers and to quantify the retinal thickness. Furthermore, OCT is also used to measure retinal blood flow and diagnose retinal edema[4] [4].
4. Classification and Treatments
DR can be classified by the clinical presentation either as non-proliferative DR (NPDR) or as proliferative DR (PDR). The first change observed in DR patients is a reduction in the retinal blood flow, which is followed by a loss of pericytes resulting in the development of micro-aneurysms, which may be associated with the appearance of retinal hemorrhages and hard exudates (Figure 1). These changes are collectively referred to as NPDR. Basement membrane thickening and leakage results in the first noticeable abnormality of NDPR. As the vascular damage progresses and a wider area of ischemia develops, neovascularization may become evident in the retina and over the optic nerve. Vascular endothelial growth factor (VEGF) is then released to develop a new nutrient supply by constructing capillary tubes. This is the stage where DR becomes PDR. These new blood vessels are fragile and tend to bleed, and cause scarring on the surface of the retina. This is the most advanced and serious form of diabetic retinopathy[11] [11].
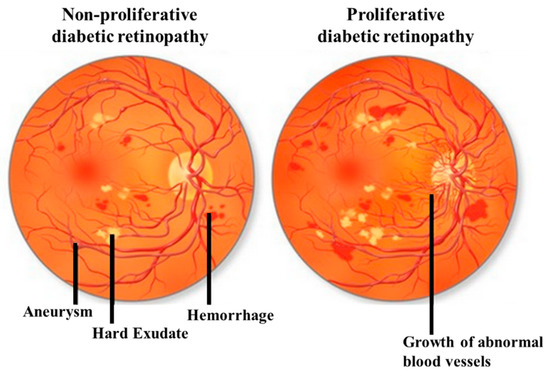
Figure 1. Illustrative pictures of non-proliferative diabetic retinopathy (DR) (NPDR) (left) and proliferative DR (PDR) (right). In early-stage NPDR, damaged blood vessels begin to leak extra fluid and blood into the eye. In PDR, many retinal blood vessels are closed, disrupting blood flow. In response to hypoxia, new blood vessels are generated (neovascularization), which are abnormal and ineffective. Illustration with permission from https://maxivisioneyehospital.wordpress.com.
At any stage of the disease, DR can be associated with diabetic macular edema (DME), DME is defined as retinal thickening caused by vascular leakage and a build-up of fluid and proteins within two-disc diameters of the macular region. DME is the major cause of severe visual impairment in diabetic patients. Diabetic macular ischemia (DMI) occurs when small blood vessels close completely over time, resulting in poor blood flow. DMI causes the death of nerve cells in the macula responsible for fine vision, which is an irreversible process resulting in a permanent, untreatable central blind spot which decreases central vision[12] [12].
NPDR and DME are considered the most sight-threatening ocular complication. Many studies have demonstrated that prevention and modification of associated systemic risk factors are the critical steps for the treatment of diabetic retinopathy. The control of blood pressure, blood glucose, and glycosylated hemoglobin levels, and lipid levels have all been associated with the reduction of the long-term risk of developing sight-threatening ocular complications. Much research has been carried out worldwide and has led to various novel therapeutic targets. The Early Treatment Diabetic Retinopathy Study (ETDRS) established pan-retinal and macular laser as the gold standard treatment for these complications[11] [11].
Laser photocoagulation and vitreoretinal surgery (vitrectomy) are the current surgical therapies that are effective in reducing the loss of vision and are useful for the late-stage disease of retinopathy but carry significant sight-threatening side effects[13] [13]. Although laser photocoagulation and pars plana vitrectomy have been shown to be useful in the treatment of severe visual loss in DR patients, visual loss continues after therapy[14] [14]. Recently, the discovery of vascular endothelial growth factor (VEGF) and its important angiogenic role has sparked new, potential therapeutic approaches. Clinical studies have demonstrated the role of VEGF in the pathogenesis of DME and exudative AMD[15][16][17] [15–17]. Damage to the retinal microvasculature results in elevated intraocular levels of VEGF, a pathophysiologic mediator in PDR and DME[18] [18]. VEGF is also associated with breakdown of the blood-retina barrier, causing increased vascular permeability which results in vascular edema. High levels of VEGF, in a different study, were found in ocular fluids of patients with PDR and DME[19] [19]. This has led to the application of anti-VEGF drugs to treat PDR and DME in combination with existing therapies. Currently, four VEGF-binding drugs including Pegaptanib, Ranibizumab, Bevacizumab, and Aflibercept (Table 1) have received U.S. Food and Drug Administration (FDA) approval for different diseases and are currently in trial for the treatment of diabetic retinopathy.
Table 1. Summary of four different anti-vascular endothelial growth factor (VEGF) drugs used in the treatment of diabetic retinopathy.
|
Name |
Trade-Name |
Description |
Clinical Trials |
|
Pegaptanib |
Macugen - Eyetech New York |
High affinity to the heparin binding site of VEGF-A isoforms. |
FDA-approved for AMD but because of disappointment visual results, only used sparingly. |
|
Ranibizumab |
Lucentis - Genentech, S. Sam Francisco |
Recombinant humanised anti-body fragment (Fab) that binds all isoforms of VEGF. |
FDA-approved for AMD, macular edema & DME. |
|
Bevacizumab |
Avastin – Genentech |
Recombinant full-length humanised monoclonal anti-body that also binds all VEGF isoforms. |
FDA-approved for rectal carcinoma, ovarian carcinoma, glioblastoma but is off set for use in ocular diseases (AMD, DME & vein occlusion). |
|
Aflibercept |
Elyea – Regeneron, Tarrytown, NY |
Recombinant fusion protein with native VEGFR ligand-binding sequences attached to the Fc segment of human IgG1. Binds all isoforms of VEGF-A, VEGF-B and placental growth factor. |
FDA-approved for AMD & macular edema and the systemic formulation Zaltrap for colorectal carcinoma. |
It is important to mention that there are also other vascular mediators that have been shown to contribute to ocular angiogenesis including members of platelet-derived growth factor family (PDGF), fibroblast growth factor family (FGF), transforming growth factor-β superfamily (TGF-β1), epidermal growth factor family (EGF), hypoxia-inducible factors insulin-like growth factors, cytokines, matrix metalloproteinases and their inhibitors, and glycosylation proteins. Recent studies investigated new drugs such as Fovista (PDGF), X-82 (VEGF & PDGF), Squalamine lactate (bFGF, PDGF& VEGF-A), and RG7716 (ANG-2 and VEGF) to target retinal neovascularization for future anti-angiogenic therapies. Full description of ongoing clinical trials of new drugs against retinal and choroidal angiogenesis are shown in Table 1 of Cabral et al.[20] [20]. Although regulating these networks of factors has recently shown to be more effective than focusing on any single one like VEGF, novel molecules should be investigated further as targets for future anti-angiogenic therapies.
Despite promising results with anti-VEGF therapy, some important issues should be considered. First, the requirement of multiple intravitreal injections can cause side effects, including cataracts, uveitis and retinal detachment[21] [21]. Furthermore, it has been reported that some patients with DR respond poorly to VEGF inhibition and, in some cases, is associated with a poor visual outcome[22][23] [22,23]. Second, current therapies are only applicable for proliferative disease and DME and aim to mitigate the results of the pathogenic process without affecting the underlying cause. Third, in a subgroup of patients with pure DMI, where small blood vessels close off completely and the retina slowly degenerates, there is absolutely no indication of using anti-VEGF drugs. VEGF has additionally been shown to influence neuronal growth and differentiation[24] [24], and to reduce the number of apoptotic retinal cells in response to ischemia, which was shown to be reversed after adding a VEGF inhibitor[25] [25]. Therefore, using anti-VEGF therapy to inhibit unwanted angiogenesis might inadvertently inhibit adult neurogenesis and neuroprotection[26] [26]. These current treatment approaches do not address the primary problems during the early stage of the disease. In these instances, regenerative medicine might introduce an alternative way to regenerate areas of vaso-degeneration and may reverse ischemia by regenerating blood vessels.
5. Vascular Tissue Engineering Technology
Vascular Tissue Engineering Technology
There are several 2D and 3D assays to develop tube-like structures. In 2D wound-healing assay, cells are cultured in a monolayer and, when cells are confluent, a “wound” is created and images captured from the beginning and at specific intervals during cell migration until the wound is closed. This method was one of the very early methods to study directional cell migration in vitro[27] [97]. More recently, the 3D aortic ring assay has been developed that is based on organ culture. In this assay, rat thoracic aorta is excised, the fat layer and adventitia removed, and small ring-like segments approximately 1mm in size are cut and embedded into a three-dimensional matrix composed of fibrin or collagen. They are maintained in a chemically defined medium, where angiogenic factors and inhibitors of angiogenesis can be directly added to the rings. In this way, new blood vessel growth and paracrine angiogenic effects can be investigated. Therefore, the aortic ring assay is a more physiologically relevant assay to study sprouting angiogenesis responses[28][29] [98,99]. The in-vitro collagen lumen assay is another 3D assay used to differentiate endothelial cells in 3D microenvironment. In this method, ECs are plated in two layers of collagen. Soon after it is formed, cells undergo morphogenesis to form a structure similar to the capillary network and appropriately polarized luminal structures[30][31] [100,101]. The third type of 3D angiogenesis assay called a “fibrin gel bead assay”, endothelial cells are coated onto cytodex microcarriers and embedded into a fibrin gel. To provide suitable factors, fibroblasts are cultured on top of the gel where they secrete factors and promote endothelial cell sprouting from the surface of the beads. After several days, many vessels are presented and can be observed under phase-contrast microscopy[32] [102]. Additionally, the retina explant assay is one of the more powerful assays for the retina. The assay involves plating a small piece of retina on an organotypic filter. In this assay, retina explants can be prepared anytime between embryonic day 13 and postnatal day 4, from which point the explants will develop very similarly to a retina in vivo and generate all of the different retinal cell types that will migrate to the appropriate layer. This assay has been shown to be very useful in the study gene function at different embryonic stages[33][34] [103,104]. All of these studies and assays illustrate that endothelial cells form vessel-like structures on Matrigel, and that they can integrate into the host tissue, forming blood vessels that were functional for 150 days when transplanted into severe combined immunodeficient mice (SCID) mice[5] [5]. Ideally, tissue-engineered vasculature that is designed for therapy must be transplantable or must stimulate vasculature formation at the transplant site. Further, engineered vessels for implantation must withstand physiological pressures without leakage or aneurysm formation, should not be thrombogenic, and should not elicit an immunological response from the patient. For clinical applications, the time that a patient must wait for a vascular therapy should be consistent with the clinical indication for use. Blood vessels range in size and include microvessels (<1 mm), small vessels (1–6 mm), and large vessels (>6 mm in diameter). Blood vessels for inducing the angiogenesis in diabetic retinopathy is categorized in microvessels, hence the focus on tissue engineering approaches in micro-scale. These approaches include stimulation of angiogenesis in vivo, by implantation of endothelial cells, or by re-endothelialization of decellularized organs. In addition, micro- fabrication technologies have recently developed as a promising approach for the future of in vivo vascular tissue engineering[35] [105].
