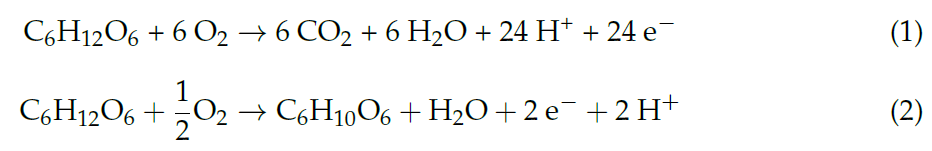Enzymatic biofuel cells can be considered as a promising solution to generate electricity from biological catalytic reactions. Indeed, enzymes show very good results as biocatalysts thanks to their excellent intrinsic properties, such as specificity toward substrate, high catalytic activity with low overvoltage for substrate conversion, mild operating conditions like ambient temperature and near-neutral pH. Furthermore, enzymes present low cost, renewability and biodegradability. The wide range of applications moves from miniaturized portable electronic equipment and sensors to integrated lab-on-chip power supplies, advanced in vivo diagnostic medical devices to wearable devices. Nevertheless, enzymatic biofuel cells show great concerns in terms of long-term stability and high power output nowadays, highlighting that this particular technology is still at early stage of development.
- Enzymatic Biofuel Cells
- Flow design
- fabrication methods
- microfluidic cell
- material engineering
1. Introduction
Due to the current environmental policies, there is a growing interest in alternative ways to produce green energy with no pollutants and emissions. Among the several developed applications, fuel cells represent a promising technology. In detail, fuel cells transform chemical reactivity into electricity by oxidizing fuel at the anode and reduce oxidant at the cathode, using noble metal catalysts, in order to provide an electrical power output, according to the fuel and oxidant availability [1]. In this research field, biofuel cells are an interesting application involving biological catalytic reactions at low temperature, in place of metal catalysts, to generate electricity from electrolysis of fuel and oxidant. Hence, a possible classification can be made considering the biological catalyst:
Nowadays EFCs are recognized as renewable and eco-friendly technologies, thanks to their peculiar features as easy miniaturization, portability, potential to produce renewable and sustainable energy [2]. Moreover, enzymatic biofuel cells present, as benefits, the possibility of operating at room temperature, high conversion efficiency, scalability and great versatility, because they can produce electrical power from a wide range of organic substrates.
Over the last decades, many studies have been carried out on the MFC and EFC development. In particular, as described in [3], MFCs possessing lifetimes of up to five years have been developed, and many of them can completely oxidize their fuel but have been limited by low current and power densities. On the other hand, EFCs show higher current and power densities, whilst limiting by fuel incomplete oxidation and lower lifetime stability. EFCs, first introduced in 1964 by Yahiro et al. [4] are expected to be better candidates as biocatalysts than microbes, not only because of their excellent intrinsic properties, such as specificity toward substrate, high catalytic activity with low overvoltage for substrate conversion, and mild operating conditions like ambient temperature and near-neutral pH, but also due to their low cost, renewability and biodegradability. Possible applications range from miniaturized portable electronic equipment, sensors to integrated lab-on-chip power supplies and advanced in vivo diagnostic medical devices that use reactants available in the ambient environment [5]. The low current density characteristic of EFCs is suitable for a wide variety of self-powered biosensors. Moreover, multiple enzymatic cells can be arranged in series or in parallel configurations, in order to generate enough energy to obtain the desired electrical parameters, thus expanding significantly the possible application areas. EFCs can be also integrated into implantable bioelectronics in living systems. An increasing interest was recently addressed to in vivo tests in many animal species, including mammals. EFCs application in human bodies through minimally invasive devices has also been investigated, reporting examples including contact lenses, which exploit some transparent and flexible materials and use lachrymal fluids, transdermal patches or even tattoos [2]. Despite all the EFCs advantages described above, research efforts are still directed to overcome the main current issues: long-term stability and high power output. Moreover, although enzymes increase the limited output performance of MFCs related to the mass transfer resistances across the cell membranes [6], their high selectivity avoids fuel complete oxidation. Several comprehensive reviews on EFCs, focusing on the materials and techniques to enhance electron transfer mechanism and the possible applications relating to wearable and implantable devices have been realized [1][2][6][7][8][9][10][11][12][13]. Nevertheless, to the best of our knowledge, no review takes into account the performance of flow-based cell designs and the most promising techniques used for electrodes and cell fabrication. Only a mini review exclusively concerning microfluidic EFCs is presented in literature to date [14]. Therefore, the main aim of this review concerns an in-depth analysis on flow-based configurations for enzymatic biofuel cells, assessing and comparing both microfluidic and non-microfluidic designs in terms of electrochemical performance and stability over the time. Specifically, a great emphasis has been placed on several promising cell fabrication methods and cell power output performance.
2. Enzymatic Fuel Cells: Fundamentals
2. Enzymatic Fuel Cells: Fundamentals
Enzymatic biofuel cells represent a particular fuel cell in which biological catalysts (enzymes) are used for fuel oxidation at the anode and oxidant reduction at the cathode [1]. An EFC can directly transform chemical energy into electricity through reactions involving biochemical steps [15]. The operating mode of an EFC resembles the functioning of conventional fuel cell: first, a fuel undergoes an enzyme-catalyzed oxidation at the anode side. This reaction releases electrons that reach the cathode side through an external circuit. In the cathode, an oxidant (usually O
) is reduced (
). Thus, the electric current flows according to a potential difference, and subsequently, an enzyme-catalyzed reaction involving a fuel (substrate) generates electrical power.
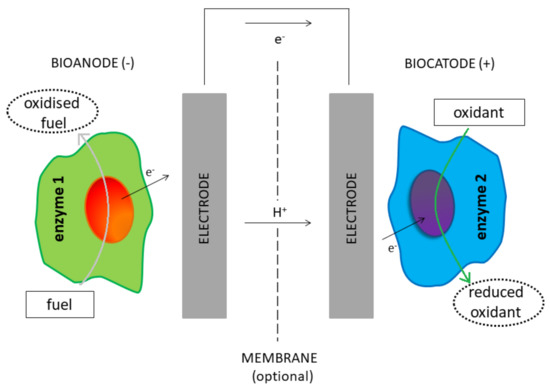
Schematic representation of an enzymatic biofuel cell (EFC) device.
In detail, as mentioned in [1], the power output of an EFC is obtained by the product of the cell voltage and the current. Cell voltages depend on many parameters, as the fuel and oxidant selected, the rate of electron transfer, the resistances within the cell (Ohmic losses) and the mass transport processes. Nowadays, the most common fuel sources are sugars. Among them the most investigated fuel is glucose, thanks to its high energy density (4430 Wh/kg) [11][16] and its wide abundance both in environment and human blood. The estimated thermodynamic reversible cell voltage for the complete oxidation of glucose to carbon dioxide and water, according to Equation (1), is 1.24 V at 298 K, considering the standard Gibbs free energies of formation of all the reaction components. This value corresponds to the maximum cell voltage expected from an enzymatic biofuel cell under standard conditions, considering a coulombic efficiency of 100% without overpotentials or ohmic losses. The complete oxidation of glucose for the extraction of 24 electrons can be only realized by multiple enzymes operating in sequences. For this reason, scientific research has mainly focused on the use of a single enzyme at the anode to oxidize glucose to gluconolactone, as shown by Equation (2), extracting only 2 electrons per mole of glucose and obtaining a maximum reversible cell voltage equal to 1.18 V. The maximum cell voltages of EFCs are commonly calculated by the difference between the formal redox potentials of the enzyme cofactors, in the active site, for anode and cathode.
Beside fully enzymatic cell devices, hybrid systems constituted by the combination of a biocatalysts at the anode and an inorganic catalyst (mainly a noble metal, as Platinum) at the cathode have also been developed. Hybrid cells produce higher power density with respect to enzymatic cell operating in the same conditions. The use of metal catalysts at the cathodic side leads to overcome the main issues related to the low activity of enzymatic biocatalysts for the oxygen reduction reaction (ORR), which constitute one of the most important limiting factors for EFCs application [17].
2.1. Substrates
Enzymatic fuel cells are able to harness power from a wide variety of renewable biological sources, employing many organic compounds derived from biomass or intermediates metabolized in living organisms. This fuel diversity represents a substantial difference between EFCs and the traditional fuel cells catalyzed by rare metals, which are predominantly powered by hydrogen or methanol. Among the broad variety of fuels potentially used in EFCs, many factors have to be taken into account in terms of EFCs development, such as their availability, possible toxicity, energy density and cost [18].
The fuels most commonly used in EFCs are different types of sugars derived from lignocellulosic biomass (xylose, fructose, sucrose and polysaccharides), since they are particularly abundant, renewable, inexpensive and safe to handle. Among them, glucose is widely employed in EFCs, due to its high theoretical energy density (4125 Wh L
), released in the case of complete oxidation to carbon dioxide and water, resulting in the production of 24 electrons per glucose molecule. Moreover, since the concentration of glucose in human blood is enough to supply an enzymatic cell, glucose-based EFCs are particularly suited for implantable applications, as potential alternatives to some traditional medical devices. The concentration of fuel is considered a key issue for a correct EFC operation: high substrate concentration is often a limiting factor for EFCs operation, due to possible severe crossover problems which lead to a decrease in system performance [18].
Other fuels are also appealing for EFCs. Renewable hydrogen produced from biomass or water splitting, characterized by one of the highest energy density values per mass, can be employed in EFCs catalyzed by hydrogenases. Methanol and ethanol derived from biomass degradation are also promising power source alternative to hydrogen in enzymatic fuel cells, thanks to some advantages, such as wide availability and low cost. Moreover, the energy densities of methanol (4047 Wh L
) and ethanol (5442 Wh L
) are, respectively, comparable and even higher than glucose. Glycerol (energy density 6260 Wh L
) is also appealing as fuel for EFCs: it is abundant, since it is a by-product of biodiesel production, and possesses important properties, as high energy density, low toxicity, low flammability and very low vapor pressure. Pyruvate (energy density 4594 Wh L
), an intermediate of the glycolysis pathway, has also been used to power EFCs. Moreover, it requires fewer enzymes than glucose for the complete oxidation to CO
and water.
References
- Leech, D.; Kavanagh, P.; Schuhmann, W. Enzymatic fuel cells: Recent progress. Electrochim. Acta 2012, 84, 223–234.
- Nasar, A.; Perveen, R. Applications of enzymatic biofuel cells in bioelectronic devices—A review. Int. J. Hydrog. Energy 2019, 44, 15287–15312.
- Cooney, M.J.; Svoboda, V.; Lau, C.; Martin, G.; Minteer, S.D. Enzyme catalysed biofuel cells. Energy Environ. Sci. 2008, 1, 320–337.
- De Poulpiquet, A.; Ciaccafava, A.; Lojou, E. New trends in enzyme immobilization at nanostructured interfaces for efficient electrocatalysis in biofuel cells. Electrochim. Acta 2014, 126, 104–114.
- Kjeang, E.; Sinton, D.; Harrington, D.A. Strategic enzyme patterning for microfluidic biofuel cells. J. Power Sources 2006, 158, 1–12.
- Ivanov, I.; Vidaković-Koch, T.; Sundmacher, K. Recent advances in enzymatic fuel cells: Experiments and modeling. Energies 2010, 3, 803–846.
- Bandodkar, A.J. Review-wearable biofuel cells: Past, present and future. J. Electrochem. Soc. 2017, 164, H3007–H3014.
- Cosnier, S.; Le Goff, A.; Holzinger, M. Towards glucose biofuel cells implanted in human body for powering artificial organs: Review. Electrochem. Commun. 2014, 38, 19–23.
- Osman, M.H.; Shah, A.A.; Walsh, F.C. Recent progress and continuing challenges in bio-fuel cells. Part I: Enzymatic cells. Biosens. Bioelectron. 2011, 26, 3087–3102.
- Holzinger, M.; Le Goff, A.; Cosnier, S. Carbon nanotube/enzyme biofuel cells. Electrochim. Acta 2012, 82, 179–190.
- Slaughter, G.; Kulkarni, T. Enzymatic Glucose Biofuel Cell and its Application. J. Biochips Tissue Chips 2015, 5.
- Datta, S.; Christena, L.R.; Rajaram, Y.R.S. Enzyme immobilization: An overview on techniques and support materials. 3 Biotech. 2013, 3, 1–9.
- Rasmussen, M.; Abdellaoui, S.; Minteer, S.D. Enzymatic biofuel cells: 30 years of critical advancements. Biosens. Bioelectron. 2016, 76, 91–102.
- Rewatkar, P.; Hitaishi, V.P.; Lojou, E.; Goel, S. Enzymatic fuel cells in a microfluidic environment: Status and opportunities. A mini review. Electrochem. Commun. 2019, 107, 106533.
- Neto, S.A.; De Andrade, A.R. New energy sources: The enzymatic biofuel cell. J. Braz. Chem. Soc. 2013, 24, 1891–1912.
- Ryu, J.; Kim, H.S.; Hahn, H.T.; Lashmore, D. Carbon nanotubes with platinum nano-islands as glucose biofuel cell electrodes. Biosens. Bioelectron. 2010, 25, 1603–1608.
- Kwon, C.H.; Ko, Y.; Shin, D.; Kwon, M.; Park, J.; Bae, W.K.; Lee, S.W.; Cho, J. High-power hybrid biofuel cells using layer-by-layer assembled glucose oxidase-coated metallic cotton fibers. Nat. Commun. 2018, 9, 1–11.
- Xiao, X.; Xia, H.; Wu, R.; Bai, L.; Yan, L.; Magner, E.; Cosnier, S.; Lojou, E.; Zhu, Z.; Liu, A.; et al. Tackling the Challenges of Enzymatic (Bio) Fuel Cells. Chem. Rev. 2019, 119, 9509–9558.