Nanobiotechnology in agriculture is a driver for modern-day smart, efficient agricultural practices. Nanoparticles have been shown to stimulate plant growth and disease resistance. The goal of sustainable farming can be accomplished by developing and sustainably exploiting the fruits of nanobiotechnology to balance the advantages nanotechnology provides in tackling environmental challenges.
- nanofertilizer
- smart delivery systems
1. Introduction
The ever-growing population coupled with shrinking cultivable agricultural areas has created an immediate and increasing demand for new technologies and processes to improve agricultural production. The concurrent demand for food calls for an increase in the production and protection of crops worldwide [1]. Despite fertilizer consumption increasing worldwide in the past few decades, the nutrient removal from soil is much higher than the nutrient additions through fertilization (N, P, and K). This has created a net negative soil nutrient balance of about 10 million tons and significantly threatened soil health, which has translated into widespread economic losses for farmers [2]. The subsequent leaching losses reduce productivity and cause associated environmental problems [3]. Thus, appropriate uses for and doses of fertilizer products need to be determined to keep the ecological impact at a minimum.
Nanobiotechnology is a revolutionary technology of the 21st century. Nanoparticles (NPs) are defined as natural or engineered small particles with a size between 1 and 100 nm which, compared to their bulk counterparts, exhibit significantly different physical and chemical properties. NPs have unique properties—including shape, size, large surface area, crystallinity, surface functionalization, porosity, zeta potential, hydrophobicity, or hydrophilicity—that allow the targeted controlled release kinetics of NPs to be used as smart delivery systems [2]. Nanoparticles offer state-of-the-art solutions for precision farming; the targeted/controlled delivery of inputs; improved soil and plant health; and, importantly, the need-based application of agricultural inputs for improved productivity, efficiency, and economic benefits. Furthermore, improved methods for the biological synthesis of agriculturally important metal NPs have come to light with the advancement and awareness of nanotechnology. Highly targeted, improved nano fertilizer formulations with a high use efficiency for plants are needed to ensure the minimal loss of nutrients [2]. This review provides a deeper understanding of the advances in nanobiotechnology in the field of agriculture, particularly biotechnological advances in nano formulation, delivery, and the fate of nano fertilizer, and encourages interactions within the scientific community for their more extensive application through innovation for sustainable agro-practices.
Nanoparticles—as a result of their small size (<100 nm), shape, atomic arrangement, and structure—have complex interactions with different biomolecules, ionic particulates, and colloids. These uncertain and complex properties of NPs make it difficult to determine their compatibility and fate in the soil–plant system. Moreover, plant–NP interaction studies are lacking due to technological gaps and efficiencies of techniques used. Comprehensive risk assessment and biocompatibility studies (cyto- and phytotoxicity) are needed for NP acceptance and to identify the fate of NPs in the soil–plant system.
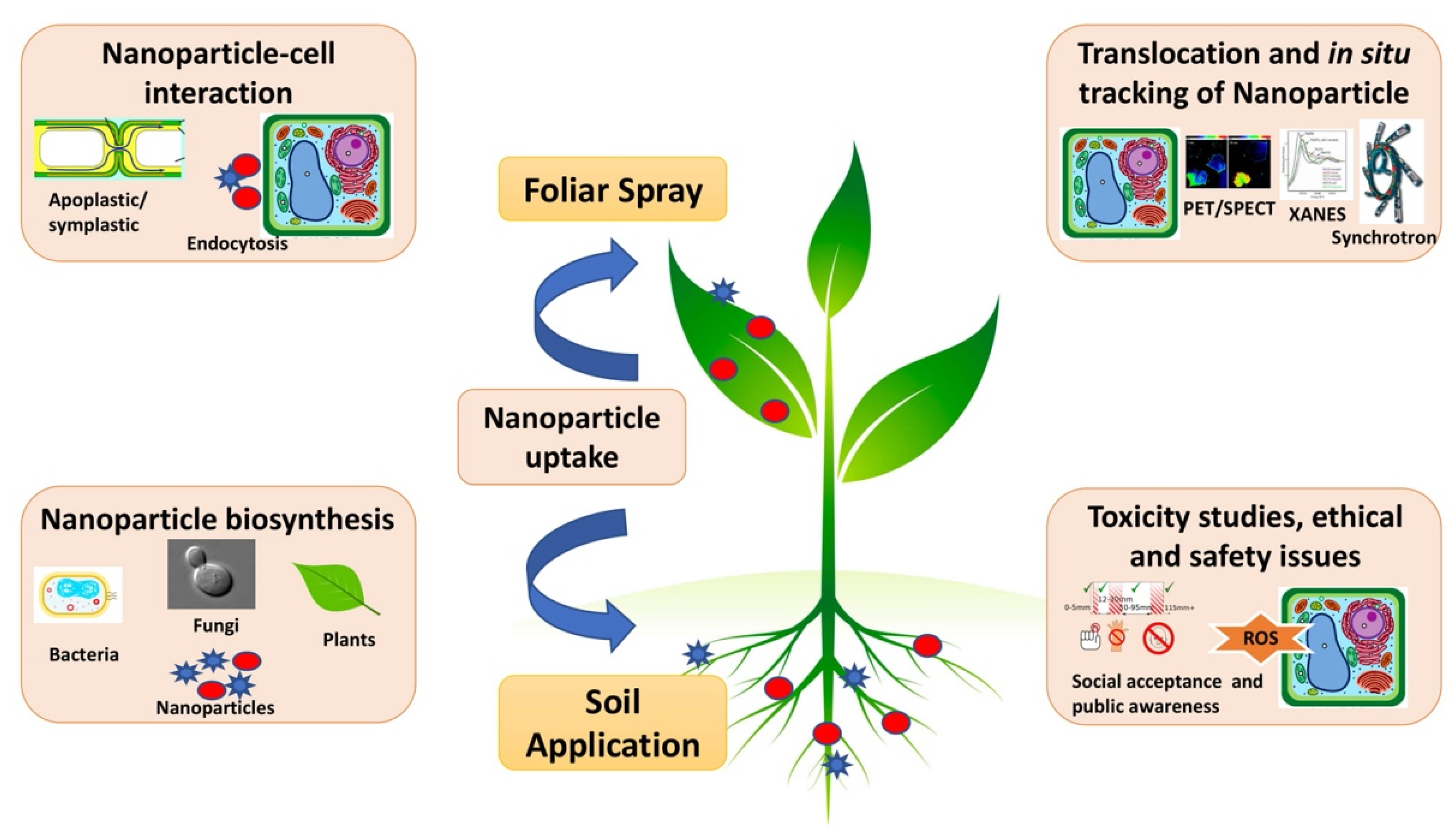
The application of nanotechnology in agriculture relies on successful implementation of regulations from all stakeholders. Budding young researchers and scientists from R&D institutes, academia, and industry from across the globe need to come together to discuss the current practices and future scope of nanotechnologies to promote innovation and knowledge transfer for agriculture. The use of agrochemicals is essential in present-day agriculture; however, there have been few advances in the fields of nanopesticides and nanofertilizers, relative to other sectors, such as food handling, storing, and packaging. Deliberations and discussions would not only promote future collaborations for accelerated product development for agricultural application but will also be captured to prepare policies that will signify the need to prioritize future research in nanotechnology, identify scope for innovation, and highlight how nano-enabled systems can address contemporary issues in agriculture for the efficient delivery of fertilizers and nutrients (Figure 1).
Figure 1.
Nanobiotechnology in agriculture for combating nutrient deficiencies with nanotoxicity challenges.
2. Advanced Use of Nanotechnology to Develop Nanofertilizers
Nanobiotechnology is a valuable tool with extensive application in ecological sustainability and has emerged as an integrated ‘toolbox’ for agro-economy and ecosystem service providers. The synthesis of NPs using living organisms—such as bacteria, fungi, and plant extracts, and their metabolites—is termed the biosynthesis of NPs. Biologically synthesized NPs or biogenic NPs are a green alternative to the existing chemical methods of NP synthesis, which are toxic and energy-intensive with hazardous byproducts that damage the environment [49,50]. Biosynthesized NPs are biocompatible, are highly reproducible, have a low polydispersity, and are easy to upscale. The biological method of NP synthesis is comparable to other existing physical or chemical methods [51]. Microorganisms, with their inherent properties to remediate and reduce heavy metals using numerous reductase enzymes, have potential as the workhorses of nanobio factories for synthesizing metallic bio-NPs [50]. Over the years, interest has moved from inorganic to biogenic NPs (e.g., nanocomposites). Nano-agrochemicals that utilize biogenic delivery frameworks compete well in terms of environmental sustainability but often do not compete economically with other agrochemicals. Investigations to determine whether nanoproducts can contend with existing chemical formulations in terms of performance and cost are required [52,53].
Nanobiotechnology is a valuable tool with extensive application in ecological sustainability and has emerged as an integrated ‘toolbox’ for agro-economy and ecosystem service providers. The synthesis of NPs using living organisms—such as bacteria, fungi, and plant extracts, and their metabolites—is termed the biosynthesis of NPs. Biologically synthesized NPs or biogenic NPs are a green alternative to the existing chemical methods of NP synthesis, which are toxic and energy-intensive with hazardous byproducts that damage the environment [4][5]. Biosynthesized NPs are biocompatible, are highly reproducible, have a low polydispersity, and are easy to upscale. The biological method of NP synthesis is comparable to other existing physical or chemical methods [6]. Microorganisms, with their inherent properties to remediate and reduce heavy metals using numerous reductase enzymes, have potential as the workhorses of nanobio factories for synthesizing metallic bio-NPs [5]. Over the years, interest has moved from inorganic to biogenic NPs (e.g., nanocomposites). Nano-agrochemicals that utilize biogenic delivery frameworks compete well in terms of environmental sustainability but often do not compete economically with other agrochemicals. Investigations to determine whether nanoproducts can contend with existing chemical formulations in terms of performance and cost are required [7][8].
The synthesis of NPs by biological means can be broadly classified into two categories—intracellular and extracellular synthesis. Intracellular synthesis occurs within the biomass/cells of plants, fungi, bacteria, etc., while extracellular synthesis occurs outside the organism in question, which is aided by several biomolecules and extracellular metabolites (such as peptides) [54]. The challenges of intracellular synthesis, including the high extraction cost and complex downstream processing, can be overcome by extracellular synthesis, which ultimately improves the efficiency of downstream applicability. In the face of bioremediation, intracellular synthesis is preferred, as it is easier to remove the microbes containing the contaminant (intracellularly or extracellularly through adsorption) [55]. For all NP synthesis methodologies, a good monodispersity can be achieved by controlling critical parameters—for example, metal salt concentration, incubation time, pH, and temperature. The biological synthesis of NPs helps in providing characteristic natural capping to NPs, thus providing additional stability which can enhance the efficacy of biological nanoparticles [50]. Several organisms known to synthesize NPs by both extracellular and intracellular strategies have been selected for intensified NP productivity due to the simple upscaling, higher surface area of reactivity, and amount of proteins produced and excreted by organisms for effective downstream handling [49].
The synthesis of NPs by biological means can be broadly classified into two categories—intracellular and extracellular synthesis. Intracellular synthesis occurs within the biomass/cells of plants, fungi, bacteria, etc., while extracellular synthesis occurs outside the organism in question, which is aided by several biomolecules and extracellular metabolites (such as peptides) [9]. The challenges of intracellular synthesis, including the high extraction cost and complex downstream processing, can be overcome by extracellular synthesis, which ultimately improves the efficiency of downstream applicability. In the face of bioremediation, intracellular synthesis is preferred, as it is easier to remove the microbes containing the contaminant (intracellularly or extracellularly through adsorption) [10]. For all NP synthesis methodologies, a good monodispersity can be achieved by controlling critical parameters—for example, metal salt concentration, incubation time, pH, and temperature. The biological synthesis of NPs helps in providing characteristic natural capping to NPs, thus providing additional stability which can enhance the efficacy of biological nanoparticles [5]. Several organisms known to synthesize NPs by both extracellular and intracellular strategies have been selected for intensified NP productivity due to the simple upscaling, higher surface area of reactivity, and amount of proteins produced and excreted by organisms for effective downstream handling [4].
In a study conducted in an inert growth medium, the application of hydroxyapatite NPs stabilized by carboxy methylcellulose increased the growth rate and seed yield of soybean by 33% and 18%, respectively [56]. However, changes in grain production due to the application of hydroxyapatite NPs were not reported in the study and no significant statistical differences were observed when comparing the application of traditional phosphorus fertilizer and hydroxyapatite NPs. It must also be considered that the results expected from real soil conditions can be different from those observed in controlled lab conditions. Recently, a nanofertilizer form of nitrogen was synthesized wherein hydroxyapatite NPs were covered with urea, leading to a slow release of nitrogen to plants. Additionally, research in rice plants has indicated that urea nanohybrids (i.e., modified hydroxyapatite) can deliver nitrogen multiple times over a period of time, much slower than synthetic urea with an enhanced yield of the grain at just half the rate utilized with synthetic urea [57]. In another study, phosphorus NPs applied as a seed treatment in a suspension of water and phosphorite increased the growth rates in nine test plants (wheat, rye, pea, barley, corn, buckwheat, tomato, radish, and cucumber) by about two-fold [58]. Similarly, the high solubility of phosphate due to the use of ammonium zeolites increased the uptake of phosphorus in plants [59]. The fungal-mediated biosynthesis of phosphorus NPs was carried out employing Aspergillius tubingensis using tri-calcium phosphate as a precursor salt [60]. The further application of biosynthesized phosphorus NPs was not reported in the study. A slow potassium release formulation of nano-potassium fertilizer was developed and found to reduce potassium losses in the soil while supporting the long-term sustained supply of potassium to plants [61]. Likewise, the foliar application of nano-potassium fertilizer improved the biomass, growth, and yield of parchment pumpkin (Cucurbita pepo) [62]. In another study, Zinc NPs improved the Zn content in grains and enhanced the growth and yield of maize growing in Zn-deficient soil [31]. However, the study was performed in sandy clay loam with a low organic matter content, and different results could be observed if the organic matter content percentage was increased even in the same soil. In another study, the use of ZnO NPs increased the lycopene content by 113% in tomato [32]. Similarly, the use of Fe NPs in various crops [39,63] improved enzyme function (heme proteins responsible for cytochromes) and overall agronomic traits, including Fe biofortification, nutritional quality, biomass, and N and P metabolism. In another study, the use of ZnO NP in Clusterbean (Cyamopsis tetragonoloba) increased the P uptake by 11%, as the Zn helps to mobilize P for plant use [32,64]. The application of ZnO NPs increased the cotton biomass and yield and increased the activity of antioxidant enzymes and other regulatory and functional proteins in soil [32,65]. Similarly, P mobilization occurred with the use of magnetite NPs (Fe
In a study conducted in an inert growth medium, the application of hydroxyapatite NPs stabilized by carboxy methylcellulose increased the growth rate and seed yield of soybean by 33% and 18%, respectively [11]. However, changes in grain production due to the application of hydroxyapatite NPs were not reported in the study and no significant statistical differences were observed when comparing the application of traditional phosphorus fertilizer and hydroxyapatite NPs. It must also be considered that the results expected from real soil conditions can be different from those observed in controlled lab conditions. Recently, a nanofertilizer form of nitrogen was synthesized wherein hydroxyapatite NPs were covered with urea, leading to a slow release of nitrogen to plants. Additionally, research in rice plants has indicated that urea nanohybrids (i.e., modified hydroxyapatite) can deliver nitrogen multiple times over a period of time, much slower than synthetic urea with an enhanced yield of the grain at just half the rate utilized with synthetic urea [12]. In another study, phosphorus NPs applied as a seed treatment in a suspension of water and phosphorite increased the growth rates in nine test plants (wheat, rye, pea, barley, corn, buckwheat, tomato, radish, and cucumber) by about two-fold [13]. Similarly, the high solubility of phosphate due to the use of ammonium zeolites increased the uptake of phosphorus in plants [14]. The fungal-mediated biosynthesis of phosphorus NPs was carried out employing Aspergillius tubingensis using tri-calcium phosphate as a precursor salt [15]. The further application of biosynthesized phosphorus NPs was not reported in the study. A slow potassium release formulation of nano-potassium fertilizer was developed and found to reduce potassium losses in the soil while supporting the long-term sustained supply of potassium to plants [16]. Likewise, the foliar application of nano-potassium fertilizer improved the biomass, growth, and yield of parchment pumpkin (Cucurbita pepo) [17]. In another study, Zinc NPs improved the Zn content in grains and enhanced the growth and yield of maize growing in Zn-deficient soil [18]. However, the study was performed in sandy clay loam with a low organic matter content, and different results could be observed if the organic matter content percentage was increased even in the same soil. In another study, the use of ZnO NPs increased the lycopene content by 113% in tomato [19]. Similarly, the use of Fe NPs in various crops [20][21] improved enzyme function (heme proteins responsible for cytochromes) and overall agronomic traits, including Fe biofortification, nutritional quality, biomass, and N and P metabolism. In another study, the use of ZnO NP in Clusterbean (Cyamopsis tetragonoloba) increased the P uptake by 11%, as the Zn helps to mobilize P for plant use [19][22]. The application of ZnO NPs increased the cotton biomass and yield and increased the activity of antioxidant enzymes and other regulatory and functional proteins in soil [19][23]. Similarly, P mobilization occurred with the use of magnetite NPs (Fe
3
O
4) in lettuce [63]. Thus, along with serving the primary role of a nano nutrient, NPs have multi-dynamic and systemic effects, with an active role in mobilizing and increasing the availability of other nutrients and improving the overall nutritional quality of farm produce [66].
) in lettuce [21]. Thus, along with serving the primary role of a nano nutrient, NPs have multi-dynamic and systemic effects, with an active role in mobilizing and increasing the availability of other nutrients and improving the overall nutritional quality of farm produce [24].
The use of nanotechnology in plant sciences is gaining interest, particularly for the use of NPs as vehicles of biomolecules of agronomic importance/agrochemicals in plants [67]. The NPs encapsulate nutrients in a nano-thin protective film or nanoemulsion, which ensures a stronghold of nutrients on the plant surface due to the higher surface tension of the nanocoating [68] and has great potential for improving crop efficiency [69]. Nanozeolites [70] and nanoclays [71] are used as soil improvement products to aid in the efficient release and retention of water and nutrients. These zeolite NPs have characteristic well-defined pore networks that ensure the slow release of the agrochemical/nutrient in question [70]. Similarly, NPs such as nanowires (including microbial nanowires) [72] and nanofibers [73] help in the development of nanosensors and related diagnostic devices for the detection of pesticides/fertilizers [74]. Hence, nanobiotechnology is a key enabling technology that could revolutionize modern agriculture.
The use of nanotechnology in plant sciences is gaining interest, particularly for the use of NPs as vehicles of biomolecules of agronomic importance/agrochemicals in plants [25]. The NPs encapsulate nutrients in a nano-thin protective film or nanoemulsion, which ensures a stronghold of nutrients on the plant surface due to the higher surface tension of the nanocoating [26] and has great potential for improving crop efficiency [27]. Nanozeolites [28] and nanoclays [29] are used as soil improvement products to aid in the efficient release and retention of water and nutrients. These zeolite NPs have characteristic well-defined pore networks that ensure the slow release of the agrochemical/nutrient in question [28]. Similarly, NPs such as nanowires (including microbial nanowires) [30] and nanofibers [31] help in the development of nanosensors and related diagnostic devices for the detection of pesticides/fertilizers [32]. Hence, nanobiotechnology is a key enabling technology that could revolutionize modern agriculture.
Various factors come into play during the uptake and translocation of NPs inside the plant system, from physiological plant factors—such as age, the plant species itself, and its biotransformation pathways—to the biophysicochemical factors of NPs, which define the functionalization of NP when presented to plant cells and have a cumulative effect on the fate of NPs [22]. The most commonly used method for providing nutrient supplements is the soil application of organic and chemical fertilizers. Soil is a dynamic and heterogeneous amalgamation of several biotic and abiotic factors (living or dead) that poses several challenges with respect to various soil properties such as texture and pH which govern the fate and longevity of fertilizer in the soil. Ion exchange capacity also plays a vital role in nutrient mobilization in the soil–plant system. Another method uses liquid fertilizers sprayed directly onto aerial plant parts, mainly leaves. This minimizes the loss of nutrients and is readily available for plant use, circumventing soil issues that render them less available to plants. Despite its clear advantages, this method requires rigorous optimization considering the role stomata and epidermal cells play in nutrient uptake, bearing in mind their diurnal physiological responses [75,76]. Another critical aspect to be taken into consideration is the size of the nutrient formulation that should not interfere or hinder the normal stomatal function by blocking the stomatal pores in case of nutrient supplied is larger than the size of the stomatal pore or if it is provided in an excess concentration.
Various factors come into play during the uptake and translocation of NPs inside the plant system, from physiological plant factors—such as age, the plant species itself, and its biotransformation pathways—to the biophysicochemical factors of NPs, which define the functionalization of NP when presented to plant cells and have a cumulative effect on the fate of NPs [33]. The most commonly used method for providing nutrient supplements is the soil application of organic and chemical fertilizers. Soil is a dynamic and heterogeneous amalgamation of several biotic and abiotic factors (living or dead) that poses several challenges with respect to various soil properties such as texture and pH which govern the fate and longevity of fertilizer in the soil. Ion exchange capacity also plays a vital role in nutrient mobilization in the soil–plant system. Another method uses liquid fertilizers sprayed directly onto aerial plant parts, mainly leaves. This minimizes the loss of nutrients and is readily available for plant use, circumventing soil issues that render them less available to plants. Despite its clear advantages, this method requires rigorous optimization considering the role stomata and epidermal cells play in nutrient uptake, bearing in mind their diurnal physiological responses [34][35]. Another critical aspect to be taken into consideration is the size of the nutrient formulation that should not interfere or hinder the normal stomatal function by blocking the stomatal pores in case of nutrient supplied is larger than the size of the stomatal pore or if it is provided in an excess concentration.
Similarly, for NPs nano agrochemicals are generally delivered to plants via three methods: seed treatment, soil application, or foliar application. In general, the possible modes of entry of NPs in aerial parts of the plants include the passive uptake of NPs that occurs through plant openings with specific size exclusion (nano/micro)—e.g., stomata, bark, hydathodes [77]. However, other physiological and anatomical perspectives should be considered to better understand how NPs and plants interact. Other viable routes for NP uptake include wound and injury on plant surfaces [78]. Lateral root junctions in the rhizodermis, especially near the root tip, provide easy access to NPs at the root level [79]. In general, the presence of microbes (symbiotic/parasitic), organic matter, and exudates, among others, in the soil complicates the dynamics of NP uptake relative to that in aboveground plant parts. Additionally, when NPs are applied to soil, rather than in a foliar spray, the high exposure of these NPs may influence soil microbial communities and agglomerate NPs due to various soil physicochemical properties that could confine NP uptake by plants [66,80,81]. It is evident from the literature that NP delivery by foliar application is advantageous for nano-nutrient uptake [22,82]. Furthermore, laboratory-scale tests have revealed that aerosol spray helps to produce monodisperse particles that do not agglomerate when applied through foliar application [66].
Similarly, for NPs nano agrochemicals are generally delivered to plants via three methods: seed treatment, soil application, or foliar application. In general, the possible modes of entry of NPs in aerial parts of the plants include the passive uptake of NPs that occurs through plant openings with specific size exclusion (nano/micro)—e.g., stomata, bark, hydathodes [36]. However, other physiological and anatomical perspectives should be considered to better understand how NPs and plants interact. Other viable routes for NP uptake include wound and injury on plant surfaces [37]. Lateral root junctions in the rhizodermis, especially near the root tip, provide easy access to NPs at the root level [38]. In general, the presence of microbes (symbiotic/parasitic), organic matter, and exudates, among others, in the soil complicates the dynamics of NP uptake relative to that in aboveground plant parts. Additionally, when NPs are applied to soil, rather than in a foliar spray, the high exposure of these NPs may influence soil microbial communities and agglomerate NPs due to various soil physicochemical properties that could confine NP uptake by plants [24][39][40]. It is evident from the literature that NP delivery by foliar application is advantageous for nano-nutrient uptake [33][41]. Furthermore, laboratory-scale tests have revealed that aerosol spray helps to produce monodisperse particles that do not agglomerate when applied through foliar application [24].
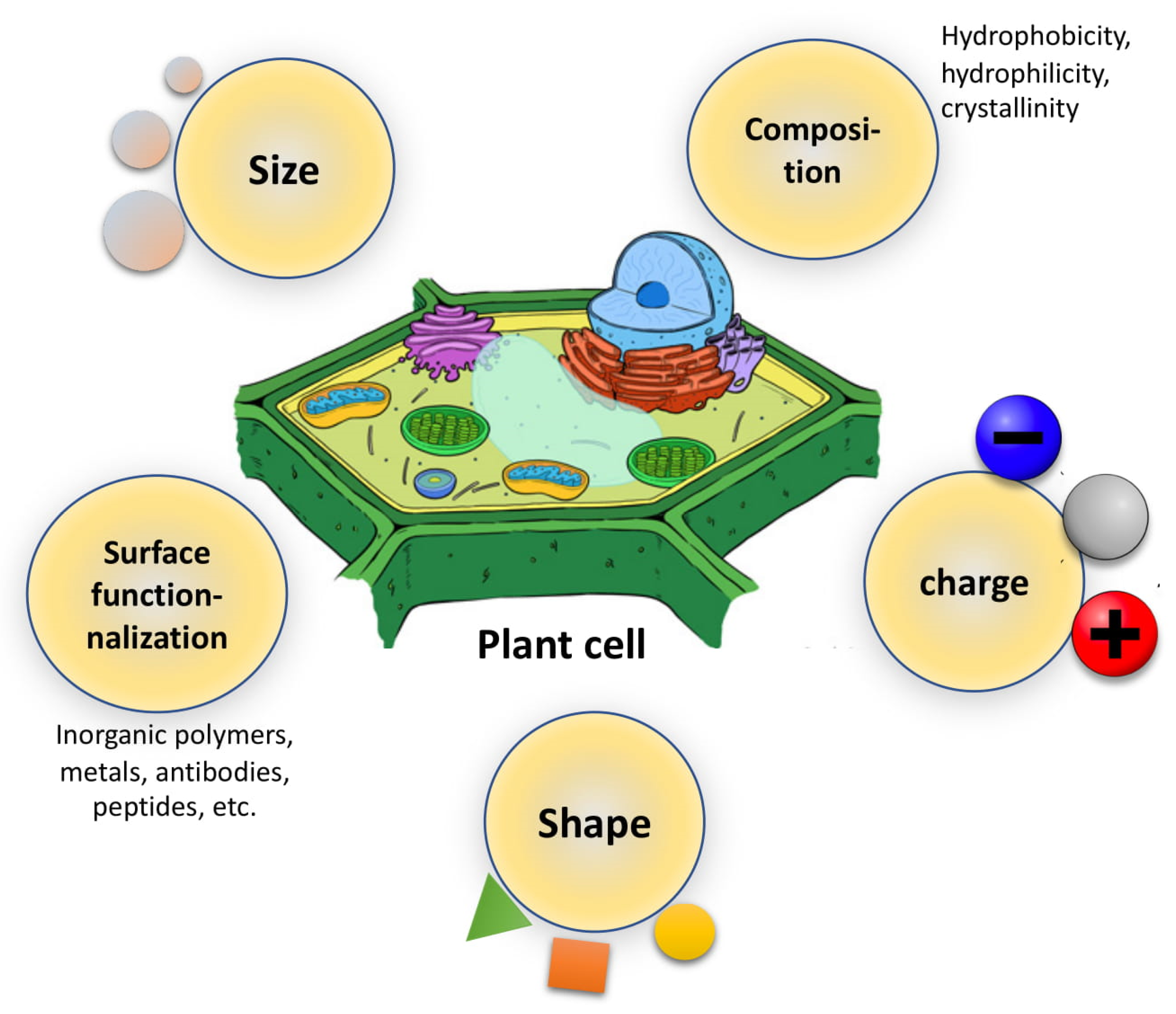
Different NP properties, such as size, shape, area of curvature, and radius, facilitate NP uptake by cells up to a threshold limit deviating from which decreases the cellular uptake (Figure 2). This might account for the difference in several contact sites in NPs of different sizes and shapes used for interaction between the NP and the cell membrane, thus affecting the free energy accessible for the NP to interact with the cell [83].
Different NP properties, such as size, shape, area of curvature, and radius, facilitate NP uptake by cells up to a threshold limit deviating from which decreases the cellular uptake (Figure 2). This might account for the difference in several contact sites in NPs of different sizes and shapes used for interaction between the NP and the cell membrane, thus affecting the free energy accessible for the NP to interact with the cell [42].
Figure 2.
Physicochemical factors of nanoparticles (NPs) affecting NPs–plant cell interaction.
References
- Rodrigues, S.M.; Demokritou, P.; Dokoozlian, N.; Hendren, C.O.; Karn, B.; Mauter, M.S.; Sadik, O.A.; Safarpour, M.; Unrine, J.M.; Viers, J. Nanotechnology for sustainable food production: Promising opportunities and scientific challenges. Environ. Sci. Nano 2017, 4, 767–781.
- Solanki, P.; Bhargava, A.; Chhipa, H.; Jain, N.; Panwar, J. Nano-fertilizers and their smart delivery system. In Nanotechnologies in Food and Agriculture; Springer: Cham, Switzerland, 2015; pp. 81–101.
- Hasler, K.; Bröring, S.; Omta, S.W.F.; Olfs, H.-W. Life cycle assessment (LCA) of different fertilizer product types. Eur. J. Agron. 2015, 69, 41–51.
- Pantidos, N. Biological Synthesis of Metallic Nanoparticles by Bacteria, Fungi and Plants. J. Nanomed. Nanotechnol. 2014, 05.
- Singh, P.; Kim, Y.J.; Zhang, D.; Yang, D.C. Biological Synthesis of Nanoparticles from Plants and Microorganisms. Trends Biotechnol. 2016, 34, 588–599.
- Wiley, B.; Herricks, T.; Sun, Y.; Xia, Y. Polyol synthesis of silver nanoparticles: Use of chloride and oxygen to promote the formation of single-crystal, truncated cubes and tetrahedrons. Nano Lett. 2004, 4, 1733–1739.
- Kah, M.; Tufenkji, N.; White, J.C. Nano-enabled strategies to enhance crop nutrition and protection. Nat. Nanotechnol. 2019, 14, 532–540.
- Kah, M. Nanopesticides and nanofertilizers: Emerging contaminants or opportunities for risk mitigation? Front. Chem. 2015, 3, 1–6.
- Hulkoti, N.I.; Taranath, T.C. Biosynthesis of nanoparticles using microbes—a review. Colloids Surf. B Biointerfaces 2014, 121, 474–483.
- Li, J.; Tian, B.; Li, T.; Dai, S.; Weng, Y.; Lu, J.; Xu, X.; Jin, Y.; Pang, R.; Hua, Y. Biosynthesis of Au, Ag and Au-Ag bimetallic nanoparticles using protein extracts of Deinococcus radiodurans and evaluation of their cytotoxicity. Int. J. Nanomed. 2018, 13, 1411.
- Liu, R.; Lal, R. Synthetic apatite nanoparticles as a phosphorus fertilizer for soybean (Glycine max). Sci. Rep. 2014, 4, 5686.
- Kottegoda, N.; Sandaruwan, C.; Priyadarshana, G.; Siriwardhana, A.; Rathnayake, U.A.; Berugoda Arachchige, D.M.; Kumarasinghe, A.R.; Dahanayake, D.; Karunaratne, V.; Amaratunga, G.A.J. Urea-Hydroxyapatite Nanohybrids for Slow Release of Nitrogen. ACS Nano 2017, 11, 1214–1221.
- Sharonova, N.L.; Yapparov, A.K.; Khisamutdinov, N.S.; Ezhkova, A.M.; Yapparov, I.A.; Ezhkov, V.O.; Degtyareva, I.A.; Babynin, E. V Nanostructured water-phosphorite suspension is a new promising fertilizer. Nanotechnol. Russ. 2015, 10, 651–661.
- Dwivedi, S.; Saquib, Q.; Al-Khedhairy, A.A.; Musarrat, J. Understanding the role of nanomaterials in agriculture. In Microbial Inoculants in Sustainable Agricultural Productivity; Singh, D.P., Singh, H.B., Prabha, R., Eds.; Springer: New Delhi, India, 2016; pp. 271–288.
- Tarafdar, J.C.; Raliya, R.; Rathore, I. Microbial synthesis of phosphorous nanoparticle from tri-calcium phosphate using Aspergillus tubingensis TFR-5. J. Bionanosci. 2012, 6, 84–89.
- Kubavat, D.; Trivedi, K.; Vaghela, P.; Prasad, K.; Vijay Anand, G.K.; Trivedi, H.; Patidar, R.; Chaudhari, J.; Andhariya, B.; Ghosh, A. Characterization of a chitosan-based sustained release nanofertilizer formulation used as a soil conditioner while simultaneously improving biomass production of Zea mays L. Land Degrad. Dev. 2020, 31, 2734–2746.
- Gerdini, F.S. Effect of nano potassium fertilizer on some parchment pumpkin (Cucurbita pepo) morphological and physiological characteristics under drought conditions. Intl. J. Farm. Alli Sci. 2016, 5, 367–371.
- Subbaiah, L.V.; Prasad, T.N.V.K.V.; Krishna, T.G.; Sudhakar, P.; Reddy, B.R.; Pradeep, T. Novel effects of nanoparticulate delivery of zinc on growth, productivity, and zinc biofortification in maize (Zea mays L.). J. Agric. Food Chem. 2016, 64, 3778–3788.
- Raliya, R.; Tarafdar, J.C. ZnO Nanoparticle Biosynthesis and Its Effect on Phosphorous-Mobilizing Enzyme Secretion and Gum Contents in Clusterbean (Cyamopsis tetragonoloba L.). Agric. Res. 2013, 2, 48–57.
- Rui, M.; Ma, C.; Hao, Y.; Guo, J.; Rui, Y.; Tang, X.; Zhao, Q.; Fan, X.; Zhang, Z.; Hou, T. Iron oxide nanoparticles as a potential iron fertilizer for peanut (Arachis hypogaea). Front. Plant Sci. 2016, 7, 815.
- Zahra, Z.; Arshad, M.; Rafique, R.; Mahmood, A.; Habib, A.; Qazi, I.A.; Khan, S.A. Metallic nanoparticle (TiO2 and Fe3O4) application modifies rhizosphere phosphorus availability and uptake by Lactuca sativa. J. Agric. Food Chem. 2015, 63, 6876–6882.
- Raliya, R.; Tarafdar, J.C. Biosynthesis and characterization of zinc, magnesium and titanium nanoparticles: An eco-friendly approach. Int. Nano Lett. 2014, 4.
- Venkatachalam, P.; Priyanka, N.; Manikandan, K.; Ganeshbabu, I.; Indiraarulselvi, P.; Geetha, N.; Muralikrishna, K.; Bhattacharya, R.C.; Tiwari, M.; Sharma, N. Enhanced plant growth promoting role of phycomolecules coated zinc oxide nanoparticles with P supplementation in cotton (Gossypium hirsutum L.). Plant Physiol. Biochem. 2017, 110, 118–127.
- Raliya, R.; Saharan, V.; Dimkpa, C.; Biswas, P. Nanofertilizer for Precision and Sustainable Agriculture: Current State and Future Perspectives. J. Agric. Food Chem. 2018, 66, 6487–6503.
- Parisi, C.; Vigani, M.; Rodríguez-Cerezo, E. Agricultural nanotechnologies: What are the current possibilities? Nano Today 2015, 10, 124–127.
- Iavicoli, I.; Leso, V.; Beezhold, D.H.; Shvedova, A.A. Nanotechnology in agriculture: Opportunities, toxicological implications, and occupational risks. Toxicol. Appl. Pharmacol. 2017, 329, 96–111.
- Sanzari, I.; Leone, A.; Ambrosone, A. Nanotechnology in Plant Science: To Make a Long Story Short. Front. Bioeng. Biotechnol. 2019, 7, 1–12.
- Jahangirian, H.; Rafiee-Moghaddam, R.; Jahangirian, N.; Nikpey, B.; Jahangirian, S.; Bassous, N.; Saleh, B.; Kalantari, K.; Webster, T.J. Green Synthesis of Zeolite/Fe2O3 Nanocomposites: Toxicity & Cell Proliferation Assays and Application as a Smart Iron Nanofertilizer. Int. J. Nanomed. 2020, 15, 1005.
- Mandal, N.; Datta, S.C.; Manjaiah, K.M.; Dwivedi, B.S.; Kumar, R.; Aggarwal, P. Evaluation of zincated nanoclay polymer composite in releasing Zn and P and effect on soil enzyme activities in a wheat rhizosphere. Eur. J. Soil Sci. 2019, 70, 1164–1182.
- Reguera, G. Harnessing the power of microbial nanowires. Microb. Biotechnol. 2018, 11, 979–994.
- Noruzi, M. Electrospun nanofibres in agriculture and the food industry: A review. J. Sci. Food Agric. 2016, 96, 4663–4678.
- Kundu, M.; Krishnan, P.; Kotnala, R.K.; Sumana, G. Recent developments in biosensors to combat agricultural challenges and their future prospects. Trends Food Sci. Technol. 2019, 88, 157–178.
- Raliya, R.; Nair, R.; Chavalmane, S.; Wang, W.-N.; Biswas, P. Mechanistic evaluation of translocation and physiological impact of titanium dioxide and zinc oxide nanoparticles on the tomato (Solanum lycopersicum L.) plant. Metallomics 2015, 7, 1584–1594.
- Alidoust, D.; Isoda, A. Effect of γFe2O3 nanoparticles on photosynthetic characteristic of soybean (Glycine max (L.) Merr.): Foliar spray versus soil amendment. Acta Physiol. Plant. 2013, 35, 3365–3375.
- Salehi, H.; Chehregani, A.; Lucini, L.; Majd, A.; Gholami, M. Morphological, proteomic and metabolomic insight into the effect of cerium dioxide nanoparticles to Phaseolus vulgaris L. under soil or foliar application. Sci. Total Environ. 2018, 616, 1540–1551.
- Kurepa, J.; Paunesku, T.; Vogt, S.; Arora, H.; Rabatic, B.M.; Lu, J.; Wanzer, M.B.; Woloschak, G.E.; Smalle, J.A. Uptake and distribution of ultrasmall anatase TiO2 Alizarin red S nanoconjugates in Arabidopsis thaliana. Nano Lett. 2010, 10, 2296–2302.
- Al-Salim, N.; Barraclough, E.; Burgess, E.; Clothier, B.; Deurer, M.; Green, S.; Malone, L.; Weir, G. Quantum dot transport in soil, plants, and insects. Sci. Total Environ. 2011, 409, 3237–3248.
- Chichiriccò, G.; Poma, A. Penetration and toxicity of nanomaterials in higher plants. Nanomaterials 2015, 5, 851–873.
- Cao, J.; Feng, Y.; Lin, X.; Wang, J. Arbuscular mycorrhizal fungi alleviate the negative effects of iron oxide nanoparticles on bacterial community in rhizospheric soils. Front. Environ. Sci. 2016, 4, 10.
- Anderson, A.; McLean, J.; McManus, P.; Britt, D. Soil chemistry influences the phytotoxicity of metal oxide nanoparticles. Int. J. Nanotechnol. 2017, 14, 15–21.
- Raliya, R.; Franke, C.; Chavalmane, S.; Nair, R.; Reed, N.; Biswas, P. Quantitative understanding of nanoparticle uptake in watermelon plants. Front. Plant Sci. 2016, 7, 1288.
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C.W. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006, 6, 662–668.