Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Maria Rosaria Plutino and Version 1 by Maria Rosaria Plutino.
The structures of these gold derivatives (i.e. gold nanoparticles, gold (I) and (III) complexes and carbene-based gold complexes) were synthesized to evaluate the influence of increased activity and/or selectivity on their pharmacological effects.
- gold derivatives
- cancer treatment
- breast cancer
- cytotoxicity
- antitumor activity
-
Introduction
- Drug delivery systems;
- Gold nanosystems;
- Gold complexes.
-
Drug Delivery Systems
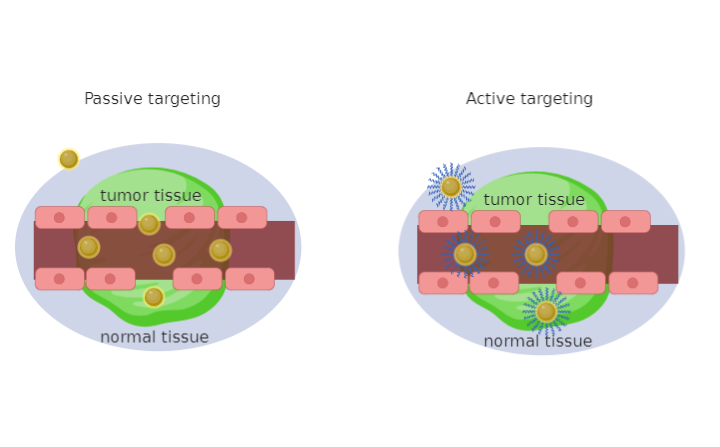
Figure 1. Schematic illustration of targeted strategies for cancer therapy by functional Gold Nanoparticles (Au-NPs, NPs=NanoParticles). Malignant tissue is distributed unevenly concerning healthy cells, hence the gold nanoparticles that cross the empty spaces due to the increase in permeability and the retention effect of passive drug release. This figure also illustrates active targeting with direct binding of gold nanoparticles to receptors with specific ligands.
1. Gold Nanosystems
Medical research on gold nanoparticles has been directed towards the study of drug delivery systems, chemotherapeutic agents, detection and diagnostics [33,34]. Gold nanosystems turned out to be attractive due to their unique properties, mainly dependent on their size and shape [35]. The resulting physical properties of nanoparticles strongly depend on the particle size, density, nature of the protective organic shell and their shape [36]. The quantum size effect occurs when the de Broglie wavelength of the valence electrons has the same dimensional order as the particle itself. Thus, the particles electronically behave like zero-dimensional quantum boxes. Therefore, the electrons are free to move inside these metal boxes with a collective oscillation frequency characteristic of plasma resonance, called the plasmon resonance band (PRB) observed near 530 nm in the 5–20 nm diameter range. The plasmon resonance of nanoparticles is closely related to the size, shape and dielectric properties of the medium surrounding the nanoparticles [37]. By varying the shape of metal nanoparticles, such as nanospheres, nanotubes, nanoprisms or core–shell nanoparticles, their optical properties vary in a quantitative and dependent manner [38]. Gold nanoparticles are used as sensors for the early diagnosis of many diseases [39]. Alzheimer’s disease and breast cancer have been targeted for essential early diagnosis [40]. With early diagnosis, current drugs have the ability to postpone the onset of symptoms typical of the disease [41] and are therefore essential for greater treatment efficacy and a higher survival rate [42]. Gold nanoshells have been used as theranostics for the diagnosis and photothermal therapy of breast cancer cells in vitro [40]. Gold nanoshells induce an important photothermal response under illumination of near infrared radiation, showing good potential for cancer therapy, with 100% efficacy in tumor remission [42,43]. The surfaces of gold nanoshells can link targeting, diagnostic and therapeutic functionalities, forming a multifunctional nanocomplex. This system has also been used in vivo, enriching the near infrared fluorescence and, at the same time, the magnetic resonance imaging capability [44]. A gold nanoparticle delivery system conjugated with gemcitabine and cetuximab as a target agent has been tested in vitro and in vivo for the treatment of pancreatic cancer cells [45]. The results of these tests showed a greater inhibition of tumor growth through a targeted system. The targeted release of multifunctional nanoparticles [46], obtained by conjugating three different peptides, was investigated: an epidermal growth factor receptor (EGFR)-recognizing peptide, an aminoterminal peptide that recognizes the urokinase plasminogen activator receptor and a peptide cyclic that recognizes the integrin receptor, to study the accumulation of gold in tumor models. These experiments did not demonstrate a substantial improvement in tumor uptake compared to control particles in vivo. Instead, gold nanoparticles with a thiolate derivative of cisplatin have been produced and tested against ovarian cancer cells [47]. The results showed that the gold conjugate with cisplatin had comparable efficacy to cisplatin alone, but toxicity to healthy cells was almost nothing, unlike the high toxicity of cisplatin used alone [48]. The decrease in toxicity towards healthy cells is one of the many reasons why therapies with gold nanoparticles can prove superior to the use of drugs alone [49]. In vivo studies have shown how multifunctional fluorescent magnetic nanocomplexes are used to trace the distribution of the nanocomplexes on tumor tissues. Nanocomplexes conjugated with specific antibodies targeting human epidermal growth factor receptor 2 (HER2) that overexpress breast cancer tumors could then be identified using magnetic resonance imaging (MRI) of the nanocomplex. As antibody-conjugated nanocomplexes are tracked throughout the body, we observe clear differences in the amounts of tumor uptake between over-expressed HER2 and low-expression HER2 tumors. This study demonstrated that it is possible to visualize tumors in vivo and that MRI could reveal a detailed picture of the distribution of nanoparticles in tumors and internal organs [50]. The diagnostic capabilities of the nanocomplexes have been visualized in vivo on HER2-expressing breast cancer tumors in animal models. Molecular targeting is achieved by combining anti-HER2 antibodies on the surface of the nanoparticle via the streptavidin-biotin binding procedure. In addition, poly ethylene glycol (PEG) conjugated to nanocomplexes is used to weaken nonspecific binding in vivo, to sterically stabilize the complexes, to implement circulation time, to lower immunogenicity and, in combination with antibodies, to increase accumulation of nanoparticles in the tumor [51]. Advantageous biological systems were investigated that exploit polyvalent interactions, allowing an organism to take advantage of a set of monovalent ligands with lower affinity, rather than using new and higher affinity monovalent ligands for each function. Ligand binding to a gold nanoparticle in the multivalent mode is an effective way to generate a high local concentration of ligands. The binding equilibria between the surface-bound ligand and the receptor can be shifted towards the formation of more ligand–receptor pairs in the presence of a high local ligand concentration according to the Le Chatelier principle [52].
One type of ligands, conjugated to gold nanoparticles, are carbazoles, extensively studied for their antioxidant and antimicrobial properties. Carbazole derivatives have also become important for their efficient inhibition of topoisomerase, tubulin, telomerase, kinase and integrase [53,54]. These compounds induce antiproliferative activity and a significant apoptotic response in a selective manner towards tumor cells [55,56,57]. The gold nanoparticles functionalized with N-thiocarbazole derivatives (Figure 2) have been employed as antiproliferative agents against breast and uterine cancer cell lines without affecting non-tumor cells [58].
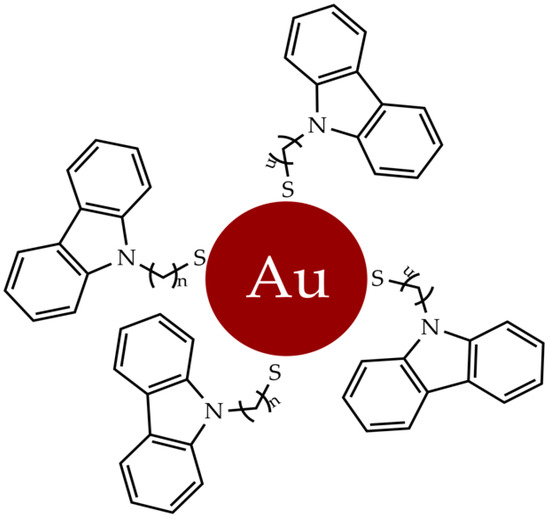
Figure 2. Gold nanoparticles functionalized with N-thioalkylcarbazole derivatives.
The unique properties of gold nanoparticles were exploited for detection assays, employing the nanoparticles as cell-permeable multivalent systems, and further investigating promising and targeted therapies for known receptors [59].
2. Gold Complexes
In recent decades, new gold complexes have been developed with antibacterial, antiviral, antiparasitic and antitumor activity [60]. Several intracellular protein targets, such as kinases, reductases, proteases and topoisomerases, interact with gold (III) complexes; in particular, the blocking of the latter leads to programmed cell death [61]. The ability of [Au(CˆNˆC)(IMe)]CF3SO3 (1) (Figure 3) to inhibit the relaxation reaction of supercoiled DNA was analyzed. By comparing the different electrophoretic mobility of both supercoiled and relaxed forms, it was shown that compound 1 was able to inhibit the relaxation activity of human IB topoisomerase.
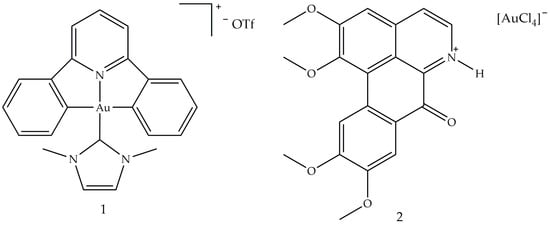
Figure 3. Molecular structure of [Au(CˆNˆC)(IMe)]CF3SO3] (1) and [OGH][AuCl4] (2) [60]. Abbreviations: CˆNˆC is the bi-cyclometallated di-anionderived from 2,6-diphenylpyridine, IMe is N,N′-dimethylimidazolium and [OGH](+) is the charged alkaloid oxoglaucine (OG).
A study conducted in 2012 described the use of oxoglaucine (OG) as a ligand, an alkaloid extract of oxoaporphine obtained from the overground parts of different plants, such as Annonaceae, Magnoliaceae and Papaveraceae [62]. The reaction was carried out between OG and a gold (III) salt, to obtain the complex of gold (III) (2), an ionic compound made up of the oxoglaucine cation and the anion [AuCl4]− having a square planar structure (Figure 3). In this compound, the oxoglaucine is protonated on the nitrogen atom while the gold (III) tetrachloride acts as a counter ion, balancing the charge. Crystallographic data confirmed that oxoglaucine organizes itself into a planar structure, capable of intercalating DNA [63]. The ability of compound 2 to block tumor growth was evaluated in vitro against different cell lines where the best results were obtained for human papillomavirus-related endocervical adenocarcinoma BEL7404 cells, with an inhibitory concentration IC50 (i.e., the half maximal inhibitory concentration) of 6.1 ± 0.5 μM and for AS49 human lung carcinoma cells (IC50 = 1.4 ± 0.7 μM) [62]. Figure 4 shows the square planar gold (III) chelates (3–6), synthesized by Wilson et al. [64], in which the pyridyl- or isoquinolylamide bidentate anionic chelators are used as ligands, in order to stabilize gold (III) through the presence of donor σ atoms. Among these, only compound 6 showed a good cytotoxic profile, towards two ovarian cancer cells (OVCAR-3 and IGROV1, with IC50 values of 4.0 and 9.8 μM, respectively) and against one colon tumor cell line (SW-620, IC50 = 15 μM). Compound 6 is both a topoisomerase IIa (TopoIIa) inhibitor, with a mechanism of action similar to that of zorubicin, and a TopoIB inhibitor, such as Topotecan and 9-methoxycamptothecin. Furthermore, this compound is structurally similar to cisplatin, but does not possess the same anticancer properties, probably due to the fact that the chloride ligands are not reactive. This condition could inhibit the replacement of water with the metal ion in vivo and the consequent formation of a gold (III)–DNA complex. Another study described the biological properties of a series of planar cationic Au3+ macrocycles containing two pyrrole-imine units linked to a quinoxaline group and an alkyl chain. Among these molecules, shown in Figure 5, compound 7 showed the best antiproliferative activity, against different types of cell lines. Moreover, it exhibits a marked inhibition activity of TopoI at 500 nM and a total inhibitor at the dose of 5 μM. In particular, the presence of gold (III) is crucial for the inhibition of topoisomerase and for DNA intercalation [65].
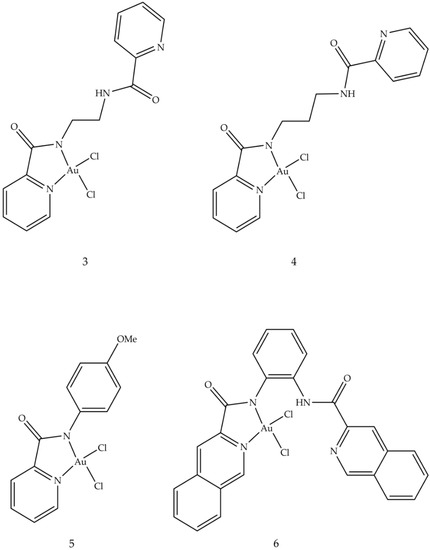
Figure 4. Molecular structures of gold (III) pyridyl and isoquinolylamido chelates (3–6) [60].
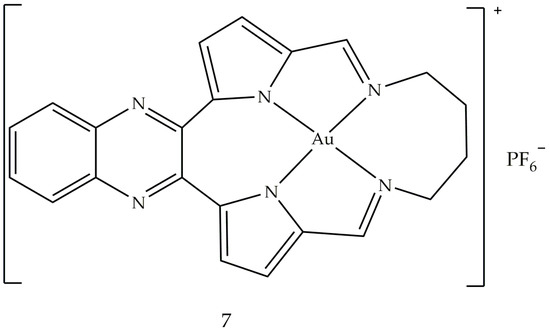
Figure 5. Molecular structure of the gold (III) macrocycle (7) [60].
Another study, conducted in 2016, ref. [66] focused on the synthesis of thiosemicarbazones coordinated with different metals (Pt, Pd and Au). Among these, the complex with the best cytotoxic activity against leukemic cells (HL-60 and THP-1, with IC50 values of 0.26 ± 0.20 and 0.62 ± 0.49 μM, respectively) and cancer cells breast (MDA-MB-231 and MCF-7, with IC50 values of 0.09 ± 0.05 and 0.42 ± 0.01 μM, respectively) resulted in [Au (PyCT4BrPh)Cl]Cl (8) (Figure 6). This compound 8 inhibits TopoIB as a function of the administered dose, with a small inhibition at 1.5 μmol L−1 and total inhibition starting at 50 μmol L−1. This result could depend on the interaction of gold with the enzyme: in fact, thiosemicarbazone not coordinated with the metal is not able to inhibit TopoIB but could be the vehicle of the metal.
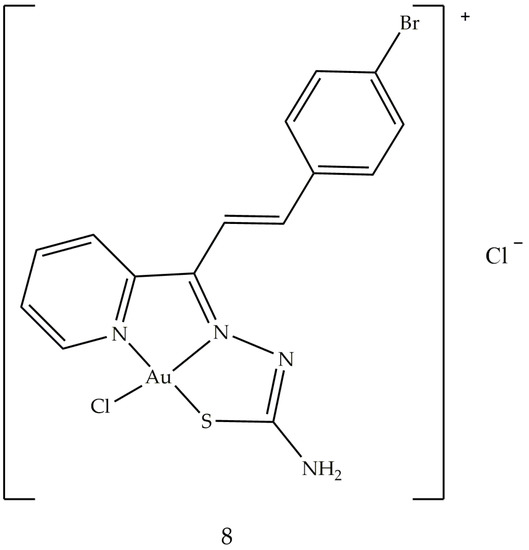
Figure 6. Molecular structure of the gold (III) thiosemicarbazone (8).
3. Gold Based Carbene Complexes
Heterocyclic and metallocene complexes have also been investigated for their potential antitumor activity [67,68,69]. These metal complexes have the general formula LnMXm, where M is the metal that constitutes the reactive center, in its oxidation state equal to 0, L is the carbene, which is the ligand able to influence the electronic properties of the metal and consequently, the possible catalytic activity of the complex and X is a non-carbenic ligand of anionic nature, such as a halide, a carboxylate or an alkoxide [70,71]. The stability and chemical reactivity of these complexes can be influenced by the covalent bonds coordinated with various transition metal centers through σ donation and π-retro-donation, the aromaticity of the NHC ligand and the volume of the side chains [72]. Among these, heterocyclic gold N-carbene (NHC) complexes attract particular interest in the pharmaceutical research sector, meeting the requirements for efficient and rapid drug design, and for thence to their chemical stability [73]. A number of gold NHC complexes have been biologically tested and, in particular, some of them have also shown anticancer effects in vitro. Au–NHC complexes offer a wide range of biological activities, such as antiarthritic [74], antimicrobial [75] and especially antitumor ones. The anticancer properties of Au (I/III)–NHC complexes have been studied in different cellular backgrounds, such as melanoma, breast, prostate and hepatocellular carcinoma cell lines. It has been shown that Au–NHC complexes can differently affect cell cycle distribution, expressions of several key regulators of apoptosis, mitochondrial integrity, activation of caspases and generation of reactive intracellular oxygen species (ROS) [76]. The structures of these gold NHC complexes were synthesized to evaluate the influence of increased lipophilicity on their pharmacological effects [77]. The lipophilic cation, whose π-electrons are delocalized, can cross biological membranes more rapidly and concentrate within the mitochondria of cancer cells. The lipophilicity of the complex increases when the nitrogen atoms are functionalized with lipophilic substituents. The effect of different substituents on the N-1 atom on pharmacological activity was tested. In particular, position 1 was functionalized with 2-cyclopentanol (L1), 2-cyclohexanol (L2) and 2-hydroxy-2-phenylethyl (L3) while in position 3 a methyl group was always present (Figure 7). The obtained complexes were studied for their anticancer properties against human breast cancer cells and the underlying molecular mechanism was studied in detail by biological assays and macromolecular docking studies, in order to understand the probable ligand–protein binding modes. One of the tested compounds, AuL3, showed good growth inhibitory activity by inducing apoptosis of breast cancer cells, without exerting any effect on healthy breast epithelial cells.
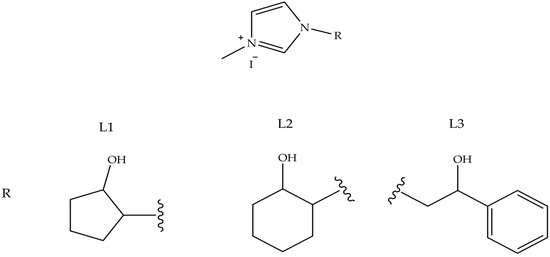
Figure 7. NH-Carbene proligands L1, L2 and L3, employed in AuLn (n = 1–3) complexes.
The antitumor activity of these complexes was evaluated on two breast cancer cell lines, MCF-7 and MDA-MB-231. The results obtained highlighted an interesting antitumor activity of the compounds shown in Figure 8, in particular on the highly aggressive and metastatic MDA-MB-231 cell line. In vitro studies have shown that these compounds are able to interfere with tubulin polymerization by inhibiting the activity of hTopo I and II, already at the minimum concentration of 1 M. Therefore, these results have shown that these compounds may be useful for further future investigations enabling the development of new multitarget agents in the treatment of breast cancer.
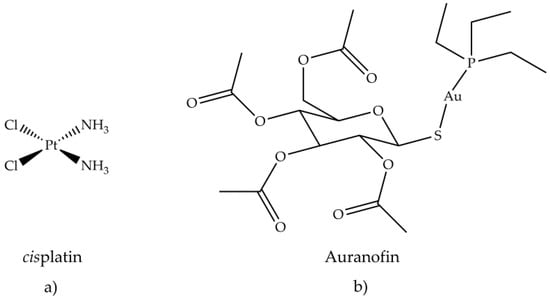
Figure 8. Chemical structure of (a) cisplatin and (b) auranofin.
Antiarthritic Gold Compounds in Oncology
The treatment of different tumors with cisplatin, (NH3)2PtCl2, has initiated and focused the pharmacological research towards metal-based drugs, such as gold (I, III) compounds, in particular, those that exhibit antiarthritic, antitumor and cytotoxic activity. The study of the effectiveness of anticancer drugs, e.g., 6-mercaptopurine and cyclophosphamide, in the treatment of rheumatoid arthritis, derived from their known immunosuppressive and anti-inflammatory actions [78,79]. This study established a connection between the two therapies, antiarthritic and antitumor. One of the reasons why scientific research has become interested in gold compounds is their square planar geometry, which unites them to platinum in cisplatin. Gold (III) is isoelectronic with platinum (II) in cisplatin and also forming similar planar square complexes. Furthermore, the biological environment is generally reducing, so gold (III) compounds can be reduced in vivo to gold (I) and metallic gold. By coordinating bioactive molecules with gold, it is possible to obtain compounds having greater activity and therapeutic effect than uncoordinated molecules. Promising in vitro inhibitory effects of auranofin (Figure 8b) against HeLa cells [80] and subsequent research on its antitumor activity were investigated. The in vitro cytotoxic activity of auranofin, tested on various cell lines, was similar or even greater than cisplatin (Figure 8a).
A good cytotoxic activity was also evaluated in vivo against P388 lymphocytic leukemia implanted in mice [81]. Several gold (I) thiolates have been tested for their anticancer activity. Aurothioglucose (Figure 9a) and aurothiomalate (Figure 9b) inhibited primary tumor growth in mice with Lewis carcinoma and reduced lung metastases [81]. Unlike cisplatin (6), aurothiomalate did not exhibit acute toxicity in vivo.
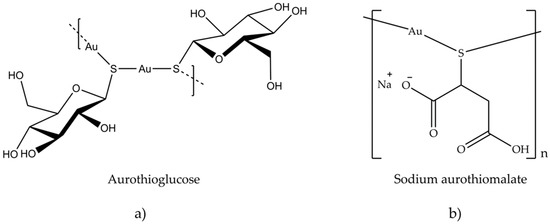
Figure 9. Chemical structure of (a) aurothioglucose and (b) sodium aurothiomalate.
The in vitro cytotoxicity of several gold compounds against B16 melanoma and P388 leukemic cells was examined in a study of auranofin and similar compounds [81]. The result shows that a wide variety of gold (I) phosphine thiolates possesses a significant cytotoxic activity. The presence of a phosphine or arsine as a binder has resulted in an increase in cytotoxicity and in vivo antitumor activity against P388 leukemia, compared to other species. The importance of the type of phosphine, used in the binuclear gold (I) compounds containing bidentate phosphines, was tested in vivo against leukemia P388, by systematically varying the nature of the phosphine and keeping the thiolate, R1S−, constant. The most active compound was the one containing (CH3CH2)[(CH3)2CH] P (Figure 10a), while the least active one had (C6H5)2P (Figure 10b).
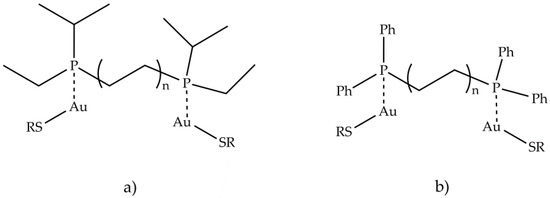
Figure 10. Structures of binuclear gold (I) compounds containing bidentate phosphines (a) (CH3CH2)[(CH3)2CH] P and (b) (C6H5)2P.
In summary, it can be stated that the most active class, among the compounds examined, was the one containing both phosphine and thioglucose ligands. The cytotoxicity and antitumor activity of several gold compounds with phosphine binders was tested and proved cytotoxic against B16 melanoma, subcutaneous breast adenocarcinoma, M5076 reticulum cell sarcoma leukemia and P388 and L1210 leukemia [82]. In addition, two new 1-acridin-9-yl-3-methylthiourea complexes Au(I), [Au(ACRTU)2]Cl (9) and [Au (ACRTU)(PPh3)]PF6 (10) (Figure 11), showed submicromolar cytotoxic activity against A2780 ovarian cancer cells and antiproliferative activity, superior to cisplatin, against some breast cancer cell lines, with a potent antiangiogenic effect. Compound 9 showed the best inhibitory effect, completely relaxing the supercoiled plasmid DNA at the concentration of 12 μM.
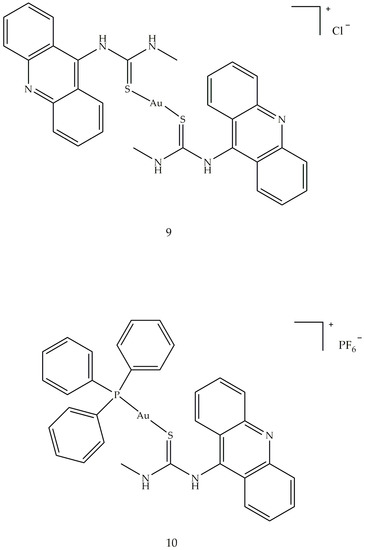
Figure 11. Molecular structure of acridine thiourea gold (I) (9 and 10).
References
- Gerald W. Prager; Sofia Braga; Branislav Bystricky; Camilla Qvortrup; Carmen Criscitiello; Ece Esin; Gabe S. Sonke; Guillem Argilés Martínez; Jean-Sebastian Frenel; Michalis Karamouzis; et al.Michiel StrijbosOzan YaziciPaolo BossiSusana BanerjeeTeresa TroianiAlexandru EniuFortunato CiardielloJosep TaberneroChristoph C. ZielinskiPaolo G. CasaliFatima CardosoJean-Yves DouillardSvetlana JezdicKeith McGregorGracemarie BricalliMalvika VyasAndré Ilbawi Global cancer control: responding to the growing burden, rising costs and inequalities in access. ESMO Open 2018, 3, e000285, 10.1136/esmoopen-2017-000285.
More
