Bone is a specialized tissue formed by different cell types and a multiscale, complex mineralized matrix. The architecture and the surface chemistry of this microenvironment can be factors of considerable influence on cell biology, and can affect cell proliferation, commitment to differentiation, gene expression, matrix production and/or composition. It has been shown that osteoblasts encounter natural motifs in vivo, with various topographies (shapes, sizes, organization), and that cell cultures on flat surfaces do not reflect the total potential of the tissue. Therefore, studies investigating the role of topographies on cell behavior are important in order to better understand the interaction between cells and surfaces, to improve osseointegration processes in vivo between tissues and biomaterials, and to find a better surface topography to enhance bone repair.
- surface topography
- osteoblasts
- cell behavior
- in vitro model
- topography sensing
- molecular transduction
- bone mineralization
Note: The following contents are extract from your paper. The entry will be online only after author check and submit it.
1. Introduction
Bone is a connective tissue of mesenchymal origin, composed of various specific cell types inside a mineralized matrix. The bone tissue is continuously remodeled through a balance between new matrix production by osteoblasts and the osteoclast-mediated resorption of the mineralized matrix. Bone repair and replacement is one of the most common medical procedures that generally requires the use of biomaterials or implants. Therefore, there is a great interest in deciphering the influence of the local microenvironment (surrounding biological tissues or implants) on the bone-producing activity of osteoblasts or the bone-resorption activity of osteoclasts. Many parameters are involved, such as the local chemical composition or rigidity. Among the relevant environmental characteristics, topography has emerged as a potent cue regulating various biological phenomena such as morphogenesis, cell migration or differentiation. In addition, topography-induced cell behaviors can be harnessed to design new, osteoconductive in vitro culture system and biomaterials. In that respect, topography-mediated effects also bear an advantage in the design of new biomaterials, as they are potentially more stable than chemistry-mediated effects such as coatings, which may be rapidly degraded by the biological environment. This review will focus on the influence of local topography on osteoblast-mediated matrix mineralization and osteogenic differentiation of mesenchymal stem cells (MSCs).
The bone presents a complex multiscale architecture [1–5][1][2][3][4][5] that has been detailed recently by 3D reconstruction of lamellar and fibro-lamellar bone tissue by FIB (Focused Ion Beam) and SEM (Scanning Electron Microscopy) [1]. The authors proposed that bone is made of ordered and disordered material, the former being predominant. They detailed nine levels of organization for both, ranging from the basic components of carbonated hydroxyapatite (HA), type I collagen molecules, water and low amounts of noncollagenous proteins at the nanometer scale (level I) to the cortical and trabecular bone at the macroscale (level IX). More recently, an extension in the number of organization levels of bone to twelve was proposed [6]. Although Petri dishes and glass substrates have been used for a long time and brought many important results [7–9][7][8][9], these flat and smooth substrates are only able to roughly reproduce up to the third or fourth level of organization proposed by Reznikov et al. [1]. The matrix produced in those culture conditions shows, at best, aligned or alternated arrays of collagen fibrils, with osteocyte-like cells trapped inside the bulk of mineralized matrix [10,11][10][11]. In particular, several in vivo histological features present in the cortical bone have never been reproduced in vitro. These include, for instance, osteon-like layered structures formed by osteoblasts over the concave surfaces left by osteoclasts after bone resorption. Thus, the osteoblasts may encounter various geometries of different scales in vivo. It is therefore important to develop in vitro model substrates with biomimicking topographies in order to study the bone-producing behavior of osteoblasts more accurately.
The reaction of osteoblasts to surface topographies is also relevant in the context of bone repair. So far, the replacement of most bone regions relies extensively on autografts. The presence of bone cells, blood vessels and calcified matrix ensures that these autografts have strong osteoinductive potential. However, the risk of morbidity and infection calls for the development of biomaterial-based synthetic grafts. To obtain biomaterials with excellent osteoinductive and osseointegrative potential, it is then important to design a biomaterial-tissue interface with the right set of parameters, including topography. As a consequence, many efforts have focused on the production of topographically structured biomaterials in order to assess their effect on bone production by osteoblasts.
2. Influence of Topography on Cells
2.1. Unordered Topographies
Examples of unordered topographies are, for example, nanoscale and microscale roughness and porosity obtained by different techniques (see Appendix A). While the morphological characteristics of roughness and porosity can be controlled experimentally, they do not necessarily present calibrated, periodical and identical geometrical patterns. Biomaterial surfaces with a given roughness or topography can be produced through dedicated mechanical or chemical procedures [12]. Results from numerous studies converge toward the consensus that rough interfaces are better for inducing osteoblast adhesion [13,14], proliferation, matrix synthesis and the production of local factors [15–17]. Titanium surfaces with microroughness or a combination of micro- and nanoroughness produced by different techniques are better at inducing the expression of osteogenic markers such as Osterix (OSX), Runx2, Osteopontin (OPN), Osteocalcin (OCN), Bone Sialoprotein (BSP), Alkaline Phosphatase (ALP) or Collagen I in osteoblasts in vitro [18–20]. This modulation by roughness of the osteogenic behavior of bone cells was confirmed on osteoblastic cells at different maturation states. Surface roughness stimulates osteogenic differentiation of less mature cells, while more mature cells are less sensitive to substrate roughness even if totally differentiated osteocyte are still affected by changes in surface roughness [17]. Interestingly, the effects of 1,25-dihydroxyvitamin D3 [1,25-OH)
2. Influence of Topography on Cells
2.1. Unordered Topographies
Examples of unordered topographies are, for example, nanoscale and microscale roughness and porosity obtained by different techniques (see Appendix A). While the morphological characteristics of roughness and porosity can be controlled experimentally, they do not necessarily present calibrated, periodical and identical geometrical patterns. Biomaterial surfaces with a given roughness or topography can be produced through dedicated mechanical or chemical procedures [12]. Results from numerous studies converge toward the consensus that rough interfaces are better for inducing osteoblast adhesion [13][14], proliferation, matrix synthesis and the production of local factors [15][16][17]. Titanium surfaces with microroughness or a combination of micro- and nanoroughness produced by different techniques are better at inducing the expression of osteogenic markers such as Osterix (OSX), Runx2, Osteopontin (OPN), Osteocalcin (OCN), Bone Sialoprotein (BSP), Alkaline Phosphatase (ALP) or Collagen I in osteoblasts in vitro [18][19][20]. This modulation by roughness of the osteogenic behavior of bone cells was confirmed on osteoblastic cells at different maturation states. Surface roughness stimulates osteogenic differentiation of less mature cells, while more mature cells are less sensitive to substrate roughness even if totally differentiated osteocyte are still affected by changes in surface roughness [17]. Interestingly, the effects of 1,25-dihydroxyvitamin D3 [1,25-OH)
2
D
3
], a bone anabolic agent used to stimulate osteoblastic differentiation, were shown to be synergistic with those of surface roughness [21]. Both mediate their effects through regulation of phospholipase A2 (PLA
2
) and activation of protein kinase A (PKA) and protein kinase C-dependent pathways which affect prostaglandin E2 (PGE
2) production [22]. Furthermore, osteoblasts cultured on rough titanium produce more
) production [22]. Furthermore, osteoblasts cultured on rough titanium produce more TGF-beta1 (TGF β1) [23] and BMP2 when cultured on microtextured titanium-based substrates [24][25]. This can explain why conditioned media from osteoblasts cultured on rough surfaces were able to drive MSCs toward an osteogenic lineage via paracrine regulation [26]. This regulation was due to signaling via α2β1 integrins in osteoblasts [27] and paracrine action of the Wnt signaling in MSCs [28][29][30][31]. These effects were higher when the osteoblasts were cultured on hydrophilic rough titanium surfaces pointing toward the potential indirect effects of roughness and hydrophilicity on cells distal from the implant site. Along with these biological signals, cell morphology and integrin signaling can also influence osteoblast differentiation and maturation. It appears that the β1 integrin subunit is involved in roughness recognition, whereas the alpha subunits are responsible for surface chemistry recognition by osteoblasts on microrough surfaces coated or not with graphitic carbon [32]. These studies show a specific integrin response to surface topography, which is essential to activate downstream signaling for osteoblast maturation [33][34].
Importantly, in vivo data confirmed that roughness not only modifies the expression of proteins but also leads to enhanced bone production. For instance, double-etched titanium implants with multiscale roughness favor in vivo osteoblast attachment and lead to an increase formation of new bone in contact with the implant [35]. Similarly, titanium implants with microroughness produced by etching lead to higher bone volume surrounding the implants. This effect was further improved by the addition of strontium at the biomaterial surface, interestingly highlighting the interest of combining a topographical and chemical modification of biomaterial interfaces [36]. Cheng and colleagues showed that implants made of rough titanium produced by sandblasting and acid etching led to a higher bone/biomaterial contact area and a higher push out force required to remove the implant, demonstrating an increased osseointegration of the biomaterial [19].
The induction of bone production by biomaterials can also be achieved through the commitment of MSCs to the osteogenic lineage. There again, roughness seems to favor the osteogenic differentiation and behavior of MSCs. These cells cultured on rough titanium produced by sandblasting and acid etching exhibit a higher ALP activity, OCN levels and Runx2 expression, although the differential remains somewhat modest [26]. Titanium etched by Reactive Ion Etching (RIE) to generate a nanoroughness also increases ALP expression of MSC cultures. Interestingly, increase in calcium and phosphate signal as measured by Energy-dispersive X-ray spectroscopy (EDX) confirmed that the osteogenic commitment of MSC results in higher deposition of mineralized matrix on the rough surface as opposed to the smooth control [37].
Recently, Ren and colleagues (2021) showed that titanium implants modified by acid etching and anodizing had similar effects both in vitro and in vivo. It enhanced cell proliferation and osteoblast differentiation in vitro, and increased bone production in vivo [38]. Chen and colleagues also found similar osteogenic results in vitro and in vivo incorporating strontium oxide to titanium surfaces [39]. These confirm that unordered surfaces can be a good inductor of osteoblast differentiation.
2.2. Ordered Topographies
The use of rough surfaces allowed us to demonstrate that nonplane surfaces favor the mineralization of matrix and bone production. To go deeper in our understanding of topography-based osteogenesis and to design optimized biomaterials, it is necessary to correlate specific topographical features such as shapes, scales, orientation or periodicity with bone production. To do so, controlled topographies with calibrated and repetitive shapes are needed. Recent progress in nano- and microfabrication has led to the fabrication of biomaterials with such controlled geometries.
Similar to what has been observed for unordered topography, the presence of ordered topography favors the production of mineralized matrix or the osteogenic differentiation compared to flat surfaces. Osteoblasts cultivated over micrometric grids show an increase in osteogenic markers such as Runx2 or OPN and an increase in calcium deposit as quantified by Alizarin Red staining [40]. Anisotropic topographies made of parallel grooves and ridges are another type of ordered topographies and are the most common of that sort in the literature. There again, the results converged to indicate that this type of geometry increases osteogenic behavior [41][42][43]. This positive effect was also confirmed in vivo as implants with grooved surfaces led to an increased volume of new bone and an increase in bone-to-implant contact area [44][45]. Interestingly, Klymov and coworkers compared a rough titanium implant with nanogrooved titanium implants and showed that the grooved surfaces where better at increasing the bone volume in the vicinity of the implant eight weeks postimplantation [44]. This could indicate that there is a specific effect of ordered topographies such as grooves and ridges over less ordered textures. However, since the processes used to produce these topographies could potentially modify their surface composition in a different way, a characterization of the surface chemistry would be necessary to assess that the effect is purely topography-based.
A strong advantage of ordered topographies is the possibility of producing a series of similar patterns with a variation of one single morphological parameter (pitch, size, shape). This allows researchers to rigorously investigate the contribution of each parameters to bone matrix production. Dhawan et al. produced surfaces with nanopores of various diameters and were able to determine an optimum level for calcium production and ALP activity at 100 nm of diameter [46]. This is consistent with the findings that titanium implants with nanodots around that size are better at promoting osteogenic differentiation and bone production. Indeed, implants decorated with nanodots in the 60–80 nm range increase the bone to implant contact area and the bone formation and reduce inflammation compared to their smooth counterparts [47][48]. The key parameters of anisotropic grooves and ridges surfaces can also be modified at will to assess their influence on osteoblast behavior. Watari et al. cultivated MSCs on grooves with widths ranging from 400 to 4000 nm and observed that the 400 nm-wide grooves had the more potent stimulation of osteogenic differentiation, with an increase expression of Runx2 and activity of ALP [43]. Width effect can also be observed at the micrometric scale. The extracellular matrix (ECM) produced in vitro by osteoblasts on micrometric grooves and ridges exhibits a higher hardness that the flat control situation, with a peak of hardness for 100 µm-wide grooves compared to on 500 µm-wide grooves [42]. OPN and OCN is increased on 50 µm-wide ridges compared to 5 µm-wide ridges [49]. So, we might consider that there is an optimal width around 50–100 µm. However, changes of other topographical parameters such as depth between studies or even between topographies of the same studies preclude definitive conclusions. Indeed, groove depth also has an impact on the osteogenic reaction of cells to topography [50]. To go deeper in the rationalization of topographical design, high throughput approaches have been implemented with the design of so-called “TopoChip”. These surfaces present a large number of randomly produced patterns from the combination of basic geometrical elements [51]. Topographies promoting thin and stretched cells, with low solidity and high compactness are better at promoting osteogenicity and this effect is mostly encountered in topography with high pattern area and low wavenumber. This systematic approach has the great advantage of predicting which kinds of patterns and which kinds of associated cellular morphologies are able to lead to higher osteogenic marker expression, matrix mineralization and implant osseointegration.
A very interesting feature of grooves and ridges is their anisotropy. Isotropic surfaces like Petri dishes, rough surfaces or microdots do not present a principal orientation. By contrast, parallel grooves and ridges define a longitudinal axis (along the grooves) and a transversal axis (across the grooves). One of the most consistent effects of contact guidance across all cell types is that cells orientate, elongate, and migrate along the longitudinal axis of anisotropic surfaces [52][53]. Reports indicate that cell alignment may lead in certain conditions to the deposition of an oriented ECM [54]. Importantly, bone is also an anisotropic tissue with a complex system of preferential orientations at different scales, and the orientation of collagens fibers and/or HA crystals is important for the mechanical properties of the mature tissues [55]. Therefore, this leads to a crucial bioengineering question: can anisotropic topography lead to the production of aligned collagen and, subsequently, oriented mineralized ECM? Osteoblasts cultivated on grooved substrates do align with the surface longitudinal axis consistently with the data known for other cell types [41][42]. However, more investigations are needed at the molecular level to determine whether the cell alignment is correlated with the alignment of collagen and/or HA crystals. Matsugaki and colleagues observed the expected alignment of primary osteoblasts with the longitudinal axis of nanometric grooves and ridges. However, they interestingly noticed a transversal alignment of collagen fibers and apatite crystals [56]. This surprising matrix orientation was further correlated with the expression of the gene tspan11 coding for a tetraspanin-family protein [57]. Cells knocked-down for tspan11 show less alignment with the surface grooves and less orthogonal orientation of collagen fibers. Unfortunately, we do not know whether HA crystals in the ECM from tspan11 knocked-down cells also lost the orthogonal orientation. Although we do not have a mechanism explaining the peculiar orientation of the mineralized matrix, these results highlight the importance of the question of topography-induced anisotropic bone matrix formation and the need for further investigations.
2.3. Scale Effect
All scales of topography are capable of inducing cell alterations, such as cell morphology, adhesion, migration, differentiation and gene expression [26][58]. However, different scales can influence the osteogenic behavior of cells in different ways, as a consequence of the cellular interaction with the surrounding environment. Nanoscale topographies mostly regulate cell behavior through spatial restrictions on the place and size of cell-substrate adhesion complexes [59]. On the other hand, cell-substrate interaction with micrometric scale topographies generate effects by modifying the entire cellular morphology [60][61][62].
Regarding the nanoscale, the literature suggests that this type of surface can modulate cell adhesion to adsorbed proteins through integrin linkage, and consequently, induce intracellular expression of specific genes [50]. Nanotopography in glass wafers has been shown to induce an increase in differentiation of hMSCs, as demonstrated by Sartori et al. [63]. Similarly, bioinspired nanostructures on titanium showed an increase in the osteogenic differentiation of hMSCs, as well as a bactericidal effect in vitro, proving their multibiofunctional properties and their potential as next-generation biomaterials for orthopedic implant [37]. Implants with nanogrooves also induce better osseointegration in rats, with an increase in trabecular and cortical bone formation in the bone face of the implant [44].
Microtopographies usually present cell-scale geometrical pattern and can induce cell deformation due to cytoskeletal remodeling, with cells adapting to substrate shape [64]. In a mouse calvaria osteoblastic cell line (MC3T3-E1) cultivated on a surface with micrometric pillars, there was an increase in osteogenic differentiation and cell proliferation compared to cells grown on a flat surface [40]. Similarly, Lagonegro et al. observed that the osteoblasts preferentially adhere to peaks on sand-blasted/acid-etched (SLA) titanium discs, whereas the concave regions presented gaps, demonstrating the importance of new studies that deal with the understanding of the cellular events involved in these cell–surface interactions [65]. Microscale is also known to improve the osseointegration of implants [38][48].
3. Molecular Mechanisms of Transduction of the Topographical Information
TGF-beta1 (TGF β1) [23] and BMP2 when cultured on microtextured titanium-based substrates [24,25]. This can explain why conditioned media from osteoblasts cultured on rough surfaces were able to drive MSCs toward an osteogenic lineage via paracrine regulation [26]. This regulation was due to signaling via α2β1 integrins in osteoblasts [27] and paracrine action of the Wnt signaling in MSCs [28–31]. These effects were higher when the osteoblasts were cultured on hydrophilic rough titanium surfaces pointing toward the potential indirect effects of roughness and hydrophilicity on cells distal from the implant site. Along with these biological signals, cell morphology and integrin signaling can also influence osteoblast differentiation and maturation. It appears that the β1 integrin subunit is involved in roughness recognition, whereas the alpha subunits are responsible for surface chemistry recognition by osteoblasts on microrough surfaces coated or not with graphitic carbon [32]. These studies show a specific integrin response to surface topography, which is essential to activate downstream signaling for osteoblast maturation [33,34].
Importantl discovery that the local geometry may carry, in vivo data confirmed that roughness not only modifies the expression of proteins but also leads to enhanced bone production. For instance, double-etched titanium implants with multiscale roughness favor in vivo osteoblast attachment and lead to an increase formation of new bone in contact with the implant [35]. Similarly, titanium implants with microroughness produced by etching lead to higher bone volume surrounding the implants. This effect was further improved by the addition of strontium at the biomaterial surface, interestingly highlighting the interest of combining a topographical and chemical modification of biomaterial interfaces [36]. Cheng and colleagues showed that implants made of rough titanium produced by sandblasting and acid etching led to a higher bone/biomaterial contact area and a higher push out force required to remove the implant, demonstrating an increased osseointegration of the biomaterial [19].
The indformation altering the behavior of cells obviouction of bone production by biomaterials can also be achieved through the commitment of MSCs to the osteogenic lineage. There again, roughness seems to favor the osteogenic differentiation and behavior of MSCs. These cells cultured on rough titanium produced by sandblasting and acid etching exhibit a higher ALP activity, OCN levels and Runx2 expression, although the differential remains somewhat modest [26]. Titanium etched by Reactive Ion Etching (RIE) to generate a nanoroughness also increases ALP expression of MSC cultures. Interestingly, increase in calcium and phosphate signal as measured by Energy-dispersive X-ray spectroscopy (EDX) confirmed that the osteogenic commitment of MSC results in higher deposition of mineralized matrix on the rough surface as opposed to the smooth control [37].
Recently, Ren and colleagues (2021) showed that titanium implants modified by acid etching and anodizing had similar effects both in vitro and in vivo. It enhanced cell proliferation and osteoblast differentiation in vitro, and increased bone production in vivo [38]. Chen and colleagues also found similar osteogenic results in vitro and in vivo incorporating strontium oxide to titanium surfaces [39]. These confirm that unordered surfaces can be a good inductor of osteoblast differentiation.
2.2. Ordered Topographies
The use of rough surfaces allowed us to demonstrate that nonplane surfaces favor the mineralization of matrix and bone production. To go deeper in our understanding of topography-based osteogenesis and to design optimized biomaterials, it is necessary to correlate specific topographical features such as shapes, scales, orientation or periodicity with bone production. To do so, controlled topographies with calibrated and repetitive shapes are needed. Recent progress in nano- and microfabrication has led to the fabrication of biomaterials with such controlled geometries.
Similar to what has been observed for unordered topography, the presence of ordered topography favors the production of mineralized matrix or the osteogenic differentiation compared to flat surfaces. Osteoblasts cultivated over micrometric grids show an increase in osteogenic markers such as Runx2 or OPN and an increase in calcium deposit as ly brings about the quantified by Alizarin Red staining [40]. Anisotropic topographies made of parallel grooves and ridges are another type of ordered topographies and are the most common of that sort in the literature. There again, the results converged to indicate that this type of geometry increases osteogenic behavior [41–43]. This positive effect was also confirmed in vivo as implants with grooved surfaces led to an increased volume of new bone and an increase in bone-to-implant contact area [44,45]. Interestingly, Klymov and coworkers compared a rough titanium implant with nanogrooved titanium implants and showed that the grooved surfaces where better at increasing the bone volume in the vicinity of the implant eight weeks postimplantation [44]. This could indicate that there is a specific effect of ordered topographies such as grooves and ridges over less ordered textures. However, since the processes used to produce these topographies could potentially modify their surface composistion of the transduction of that information in a different way, a characterization of the surface chemistry would be necessary to assess that the effect is purely topography-based.
A strong advantage of ordered topographies is the possibito biolity of producing a series of similar patterns with a variation of one single morphologigical parameter (pitch, size, shape). This allows researchers to rigorously investigate the contribution of each parameters to bone matrix production. Dhawan et al. produced surfaces with nanopores of various diameters and were able to determine an optimum level for calcium production and ALP activity at 100 nm of diameter [46]. This is consistent with the findings that titanium implants with nanodots around that size are better at promoting osteogenic differentiation and bone production. Indeed, implants decorated with nanodots in the 60–80 nm range increase the bone to implant contact area and the bone formation and reduce inflammation compared to their smooth counterparts [47,48]. The key parameters of anisotropic grooves and ridges surfaces can also be modified at will to assess their influence on osteoblast behavior. Watari et al. cultivated MSCs on grooves with widths ranging from 400 to 4000 nm and observed that the 400 nm-wide grooves had the more potent stimulation of osteogenic differentiation, with an increase expression of Runx2 and activity of ALP [43]. Width effect can also be observed at the micrometric scale. The extracellular matrix (ECM) produced in vitro by osteoblasts on micrometric grooves and ridges exhibits a higher hardness that the flat control situation, with a peak of hardness for 100 µm-wide grooves compared to on 500 µm-wide grooves [42]. OPN and OCN is increased on 50 µm-wide ridges compared to 5 µm-wide ridges [49]. So, we might consider that there is an optimal width around 50-100 µm. However, changes of other topographical parameters such as depth between studies or even between topographies of the same studies preclude definitive conclusions. Indeed, groove depth also has an impact on the osteogenic reaction of cells to topography [50]. To go deeper in the rationalization of topographical design, high throughput approaches have been implemented with the design of so-called “TopoChip”. These surfaces present a large number of randomly produced patterns from the combination of basic geometrical elements [51]. Topographies promoting thin and stretched cells, with low solidity and high compactness are better at promoting osteogenicity and this effect is mostly encountered in topography with high pattern area and low wavenumber. This systematic approach has the great advantage of predicting which kinds of patterns and which kinds of associated cellular morphologies are able to lead to higher osteogenic marker expression, matrix mineralization and implant osseointegrationthways. The presence of roughness affects the morphology and spreading of osteoblasts.
A very interesting feature of grooves and ridges is their anisotropy. Isotropic surfaces like Petri dishes, rough surfaces or microdots do not present a principal orientation. By contrast, parallel grooves and ridges define a longitudinal axis (along the grooves) and a transversal axis (across the grooves). One of the most consistent effects of contact guidance across all cell types is that cells orientate, elongate, and migrate along the longitudinal axis of anisotropic surfaces [52,53]. Reports indicate that cell alignment may lead in certain conditions to the deposition of an oriented ECM [54]. Importantly, bone is also an anisotropic tissue with a complex system of preferential orientations at different scales, and the orientation of collagens fibers and/or HA crystals is important for the mechanical properties of the mature tissues [55]. Therefore, this leads to a crucial bioengineering question: can anisotropic topography lead to the production of aligned collagen and, subsequently, oriented mineralized ECM? Osteoblasts cultivated on grooved substrates do align with the surface longitudinal axis consistently with the data known for other cell types [41,42]. However, morendeed, osteoblasts spread less on rough titanium investigations are needed at the molecular level to determine whether the cell alignment is correlated with the alignment of collagen and/or HA crystals[65][66]. Matsugaki and colleagues observed the expected alignment of primary osteoblasts with the longitudinal axis of nanometric grooves and ridges. However, they interestingly noticed a transversal alignment of collagen fibers and apatite crystals [56]. This surprising matrix orientation was further correlated with the expression of the gene tspan11 coding for a tetraspanin-family protein [57]. Cells knocked-down for tspan11 show less alignment with the surface grooves and less orthogonal orientation of collagen fibers. Unfortunately, we do not know whether HA crystals in the ECM from tspan11 knocked-down cells also lost the orthogonal orientation. Although we do not have a mechanism explaining the peculiar orientation of the mineralized matrix, these results highlight the importance of the question of topography-induced anisotropic bone matrix formation and the need for further investigations.
2.3. Scale Effect
All scales of topography are capable of inducing cell alterations, such as cell morphology, adhesion, migration, differentiation and gene expression [26,58]. However, different scales can influence the osteogenic behavior of cells in different ways, as a consequence of the cellular interaction with the surrounding environment. Nanoscale topographies mostly regulate cell behavior through spatial restrictions on the place and size of cell-substrate adhesion complexes [59]. On the other hand, cell-substrate interaction with micrometric scale topographies generate effects by modifying the entire cellular morphology [60–62].
Regarding the nanoscale, the literature suggests that this type of surface can modulate cell adhesion to adsorbed proteins through integrin linkage, and consequently, induce intracellular expression of specific genes [50]. Nanotopography in glass wafers has been shown to induce an increase in differentiation of hMSCs, as demonstrated by Sartori et al. [63]. Similarly, bioinspired nanostructures on titanium showed an increase in the osteogenic differentiation of hMSCs, as well as a bactericidal effect in vitro, proving their multibiofunctional properties and their potential as next-generation biomaterials for orthopedic implant [37]. Implants with nanogrooves also induce better osseointegration in rats, with an increase in trabecular and cortical bone formation in the bone face of the implant [44].
Microtopographies usually present cell-scale geometrical pattern anlso exhibit an altered can induce cell deformation due to cytoskeletal remodeling, with cells adapting to substrate shape [64]. In a mouse calvaria osteoblastic cell line (MC3T3-E1) cultivated on a surface with micrometric pillars, there was an increase in osteogenic differentiation and cell proliferation compared to cells grown on a flat surface [40]. Similarly, Lagonegro et al. observed that the osteoblasts preferentially adhere to peaks on sand-blasted/acid-etched (SLA) titanium discs, whereas the concave regions presented gaps, demonstrating the importance of new studies that deal with the understanding of the cellular events involved in these cell–surface interactions [65]. Microscale is also known to improve the osseointegration of implants [38,48].
3. Molecular Mechanisms of Transduction of the Topographical Information
Thhape with smaller and more elongate discovery that the local geometry may carry information altering the behavior of cells obviously brings about the question of the transduction of that information into biological pathways. The presence of roughness affects the morphology and spreading of osteoblasts. Indeed, osteoblasts spread less on rough titanium [65,66]. MSCs also exhibit an altered shape with smaller and more elongated cells on rough sur cells on rough surfaces [37]. Topography-induced changes in cell shape or elongation are common features of the transduction of topography observed in almost any cell type [67] and generally connect with changes in the cell focal adhesions (FA) and cytoskeleton organization [59,68][59][68].
The modification in integrin mediated adhesion and force generation in response to specific topographies appear to be a key step in the transduction of the topographic signal. Osteogenic surfaces such as micropits, microgrids or microroughness increase the number and/or the size of FA [40[40][49][69][70][71],49,69–71], with larger FA being indicative of stronger adhesion and traction forces. Conversely, nonosteogenic topographies like ordered nanopits or micrometric grooves and ridges decreases the number or size of FA [72–74][72][73][74]. The importance of adhesion size is well illustrated by the fact that the impaired osteogenicity of nanogrooves is correlated with the shift of adhesions from large FA to the smaller and more transient focal complexes [75]. These integrin-based adhesions are not mere points of anchorage onto the substrates. Rather, they work as mechanosensitive signaling hubs whose activity can be modulated by the topography (Figure 1). Indeed, osteogenesis-inhibiting topographies like ordered nanopits decrease the expression of the Focal Adhesion Kinase (FAK) [74] while osteogenic surfaces such as micrometric grooves and ridges, nanoroughness or titanium nanotubes increase the amount of phosphorylated FAK, a read-out generally associated with increase FA signaling [40,70,76][40][70][76]. The topography-induced changes in adhesion can be explained in part by a direct effect of topography on the level of expression of key integrins. Indeed, osteogenic nanorough surfaces increase the expression of integrin αV, α2 and β1, β3 and β5 [26,29,70,77,78][26][29][70][77][78]. Importantly, the knock-down of integrin αV, β3 or α2 decreases the expression of osteogenic markers, demonstrating the key role of upstream integrin mediated adhesion in the transduction of the topographical cue [26,77,78][26][77][78]. This central role of integrin-mediated adhesion in topography sensing strongly suggests that topography sensing is a specific case of the better known mechanosensing, involved, for instance, in the shear stress-induced or stiffness-induced osteogenicity [79,80][79][80].
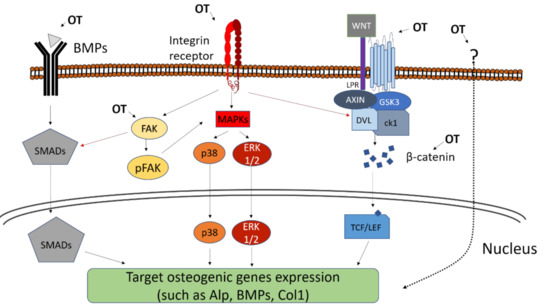
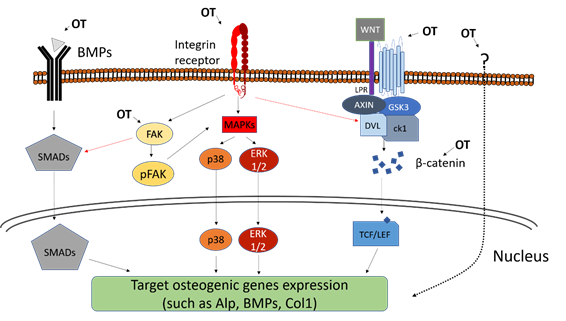
Downstream of integrins, the MAPK pathways, such as the ERK pathway or the p38 pathway, can be activated (Figure 1). They are essential to bone production, as mice expressing constitutively active ERK present Runx2-dependent enhanced bone production in vivo contrary to mice expressing dominant negative forms of ERK [81]. The topography-induced integrin signaling (shown by the increased amount of pFAK) and osteogenicity (illustrated by osteogenic marker expression) are correlated with the upregulation of MAPK-related genes [73,82,83][73][82][83]. Consistently, the phosphorylation of ERK increases on osteogenic topographies [76,84][76][84] and the inhibition of ERK and p38 hampers further osteogenic behavior.
Another important pathway in the transduction of topographical cues into matrix mineralization are the BMPs (Figure 1). BMPs bind to cell surface BMP receptors and eventually activate transcription factors of the Smad Family [85] that in turn induce the expression of genes involved in osteoblastogenesis and bone formation, such as Runx2 and OSX. The presence of osteogenic topographies increases the expression of BMP2, BMP6 [86,87][86][87] as well as BMP receptors such as BMP2R, suggesting that the BMP pathway may be triggered by the topographical cue. Consistently, BMP expression is associated with the increase in osteogenic markers such as Runx2, OSX, OPN, OCN, BSP and ALP activity and calcium deposition [87]. The importance of BMP in topography transduction is also illustrated by the reverse situation: topographies inhibiting osteogenic differentiation of MSCs lead to a downregulation of BMP receptors and a consistent decrease in Runx2 expression [75]. Importantly, the knock-down of the BMP receptor BMPR1A diminishes the osteogenic effect of nanorough surface [86]. Altogether, this demonstrates that the osteogenic effect of topography is mediated in part by the activation of the BMP pathway and the subsequent upregulation of osteogenesis-related genes such as Runx2. In addition of triggering the BMP pathway via the upregulation of BMP and BMP receptor expression, topographies can also enhance cell sensitivity to already present BMPs. For instance, the osteogenic effect of BMP9 gets stronger when MSC are cultured on rough substrates [88]. Although strong lines of evidence show that BMP are important in the osteogenic reaction to topography, the initial step causing the topography-induced increase in BMP expression remains unclear. To that respect, it is interesting to note that the BMP receptor BMPR1 and the integrin αV β5 tend to cluster over osteogenic nanopit topography [89]. The inhibition of BMPR1 decreases the integrin chains’ expression, suggesting a crosstalk between adhesion and BMP signaling. It is thus likely that the modification of integrin-based adhesions by the topography is also the starting point of the BMP-mediated response to topography.
The Wnt pathway is another pathway heavily involved in osteoblasts’ maturation [90] (Figure 1). Nanoroughness induces the expression of several Wnt ligands including Wnt3a, Wnt5a and Wnt11, as well as Wnt-related transcription factors such as NFATs and the Wnt receptors of the Frizzled family [87,91,92][87][91][92]. As a consequence, the downstream Wnt effector beta-catenin is increasingly translocated to the nucleus in MSCs cultivated over rough surfaces [91,93][91][93]. In all those conditions, the increased signaling through Wnt and beta-catenin correlates with expression of osteogenic markers and calcium deposition. Consistently, some studies using the MG63 osteoblast cell line cultivated on nanotextured titanium reported the diminution of the Wnt inhibitor DKK2, whereas exogenous addition of the inhibiting factor DKK1 downregulates the downstream expression of the osteogenic markers ALP, BMP and Collagen I [92]. As well as the BMP signaling, it is likely that integrin-mediated adhesion is involved at the starting point. Indeed, the silencing of the integrin β3 not only blocks the osteogenic behavior of MSCs cultivated on nanoroughness but also decreases the expression of elements from the Wnt pathway such as beta-catenin or Frizzled 5 [77]. Consistently, osteogenic rough surfaces also increase the expression of the Wnt ligand Wnt5a in MSC together with the expression of the integrins α1, α2 and αV [29]. Importantly, the exogenous addition of Wnt5a and Wnt5a knock-down experiments demonstrated that the integrin upregulation was Wnt-dependent [29] and suggested a mutually reinforcing crosstalk between integrin adhesions and Wnt signaling.
The aforementioned pathways (ERK, BMP, Wnt) have in common their strong ties with the remodeling of integrin-mediated adhesions in contact with topography. This suggests that the transduction of the topographical information starts at the cell-substrate interface and might come down to the question of the number, distribution and composition of the adhesion sites. However, topography can be sensed by the cell as a whole owing to the morphological deformation it imposes. MSCs growing on micrometric grooves and ridges tend to align and elongate in the direction of the grooves. This leads to a stretching and orientation of the nuclei [69]. Some studies using ordered nanodots or micrometric grooves and ridges show that nuclear deformation was associated with the reorganization of some chromosomic territories and alteration in the gene expression from the affected chromosomes [94,95][94][95]. Interestingly, MSCs growing on surfaces with cell-scale convex or concave curvature present morphological modifications of the nuclei, where convexity leads to a flattening and bending of nuclei [96,97][96][97]. This is accompanied with an increase in the level of lamin in the nuclear envelope and an enhanced osteogenic differentiation as illustrated by a higher production of OCN. In those cases, the entire cell is responsible for the sensing of the surrounding topographies. Given that the adhesion-mediated topography sensing involves the same signaling pathways classically associated with osteogenic differentiation, the molecular events are fairly well understood. By contrast, deformation-induced topography sensing remains unclear, especially because it involves the whole cell as a unique and complex mechanical unit and thus represents an enticing future challenge for the field.
Figure 1. Schematic figure of the topography-induced osteogenesis. Some pathways and proteins are shown to be related to this activation, including MAPK pathway, Focal Adhesion Kinase (FAK) activation, BMPs and the Wnt/b-catenin pathway. An osteogenic topography (OT) can activate these pathways in several ways, such as: (1) in the MAPK pathway, activation occurs from the connection to integrins, leading to activation and translocation to the nucleus of p38 and ERK ½; (2) BMPs, when connecting to a BMP receptor, trigger the activation and translocation of SMADs; (3) and the Wnt protein complex coupled to its specific receptor trigger the accumulation of beta-catenin and its translocation to the nucleus. Other forms of topography-induced osteogenic phenotype are not yet fully elucidated (dotted arrow), but it is known that the activation of the FAK and beta-catenin pathways may be involved and be activated through other pathways not yet known.
References
- Reznikov, N.; Shahar, R.; Weiner, S. Bone hierarchical structure in three dimensions. Acta Biomater. 2014, 10, 3815–3826.
- Landis, W.J.; Jacquet, R. Association of calcium and phosphate ions with collagen in the mineralization of vertebrate tissues. Calcif. Tissue Int. 2013, 93, 329–337.
- Rho, J.Y.; Kuhn-Spearing, L.; Zioupos, P. Mechanical properties and the hierarchical structure of bone. Med. Eng. Phys. 1998, 20, 92–102.
- Weiner, S.; Wagner, H.D. The Material Bone: Structure-Mechanical Function Relations. Annu. Rev. Mater. Sci. 1998, 28, 271–298.
- Zenzes, M.; Bortel, E.L.; Fratzl, P.; Mundlos, S.; Schuetz, M.; Schmidt, H.; Duda, G.N.; Witte, F.; Zaslansky, P. Normal trabecular vertebral bone is formed via rapid transformation of mineralized spicules: A high-resolution 3D ex-vivo murine study. Acta Biomater. 2019, 86, 429–440.
- Reznikov, N.; Bilton, M.; Lari, L.; Stevens, M.M.; Kroger, R. Fractal-like hierarchical organization of bone begins at the nanoscale. Science 2018, 360.
- Gerstenfeld, L.C.; Chipman, S.D.; Kelly, C.M.; Hodgens, K.J.; Lee, D.D.; Landis, W.J. Collagen expression, ultrastructure assembly, and mineralization in cultures of chicken embryo osteoblasts. J. Cell Biol. 1988, 106, 979–989.
- Gremare, A.; Aussel, A.; Bareille, R.; Paiva Dos Santos, B.; Amedee, J.; Thebaud, N.B.; Le Nihouannen, D. A Unique Triculture Model to Study Osteoblasts, Osteoclasts, and Endothelial Cells. Tissue Eng. Part C Methods 2019, 25, 421–432.
- Aureille, J.; Buffiere-Ribot, V.; Harvey, B.E.; Boyault, C.; Pernet, L.; Andersen, T.; Bacola, G.; Balland, M.; Fraboulet, S.; Van Landeghem, L.; et al. Nuclear envelope deformation controls cell cycle progression in response to mechanical force. EMBO Rep. 2019, e48084.
- Barragan-Adjemian, C.; Nicolella, D.; Dusevich, V.; Dallas, M.R.; Eick, J.D.; Bonewald, L.F. Mechanism by which MLO-A5 late osteoblasts/early osteocytes mineralize in culture: Similarities with mineralization of lamellar bone. Calcif. Tissue Int. 2006, 79, 340–353.
- Querido, W.; Abracado, L.G.; Rossi, A.L.; Campos, A.P.; Rossi, A.M.; San Gil, R.A.; Borojevic, R.; Balduino, A.; Farina, M. Ultrastructural and mineral phase characterization of the bone-like matrix assembled in F-OST osteoblast cultures. Calcif. Tissue Int. 2011, 89, 358–371.
- Anselme, K.; Bigerelle, M. Role of materials surface topography on mammalian cell response. Int. Mater. Rev. 2011, 56, 243–266.
- Bigerelle, M.; Anselme, K.; Noël, B.; Ruderman, I.; Hardouin, P.; Iost, A. Improvement in the morphology of surfaces for cell adhesion: A new process to double human osteoblast adhesion on Ti-based substrates. Biomaterials 2002, 23, 1563–1577.
- Anselme, K.; Bigerelle, M. Topography effects of pure titanium substrates on human osteoblast long-term adhesion. Acta Biomater. 2005, 1, 211–222.
- Martin, J.Y.; Schwartz, Z.; Hummert, T.W.; Schraub, D.M.; Simpson, J.; Lankford, J., Jr.; Dean, D.D.; Cochran, D.L.; Boyan, B.D. Effect of titanium surface roughness on proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63). J. Biomed. Mater. Res. 1995, 29, 389–401.
- Links, J.; Boyan, B.D.; Blanchard, C.R.; Lohmann, C.H.; Liu, Y.; Cochran, D.L.; Dean, D.D.; Schwartz, Z. Response of MG63 osteoblast-like cells to titanium and titanium alloy is dependent on surface roughness and composition. Biomaterials 1998, 19, 2219–2232.
- Lohmann, C.H.; Bonewald, L.F.; Sisk, M.A.; Sylvia, V.L.; Cochran, D.L.; Dean, D.D.; Boyan, B.D.; Schwartz, Z. Maturation state determines the response of osteogenic cells to surface roughness and 1,25-Dihydroxyvitamin D3. J. Bone Miner. Res. 2000, 15, 1169–1180.
- Gittens, R.A.; McLachlan, T.; Olivares-Navarrete, R.; Cai, Y.; Berner, S.; Tannenbaum, R.; Schwartz, Z.; Sandhage, K.H.; Boyan, B.D. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials 2011, 32, 3395–3403.
- Cheng, M.; Qiao, Y.; Wang, Q.; Jin, G.; Qin, H.; Zhao, Y.; Peng, X.; Zhang, X.; Liu, X. Calcium Plasma Implanted Titanium Surface with Hierarchical Microstructure for Improving the Bone Formation. ACS Appl. Mater. Interfaces 2015, 7, 13053–13061.
- Yin, C.; Zhang, Y.; Cai, Q.; Li, B.; Yang, H.; Wang, H.; Qi, H.; Zhou, Y.; Meng, W. Effects of the micro-nano surface topography of titanium alloy on the biological responses of osteoblast. J. Biomed. Mater. Res. A 2017, 105, 757–769.
- Boyan, B.D.; Batzer, R.; Kieswetter, K.; Liu, Y.; Cochran, D.L.; Szmuckler-Moncler, S.; Dean, D.D.; Schwartz, Z. Titanium surface roughness alters responsiveness of MG63 osteoblast-like cells to 1a,25-(OH)2D3. J. Biomed. Mater. Res. 1998, 39, 77–85.
- Schwartz, Z.; Lohmann, C.H.; Sisk, M.A.; Cochran, D.L.; Sylvia, V.L.; Simpson, J.; Dean, D.D.; Boyan, B.D. Local factor production by MG63 osteoblast-like cells in response to surface roughness and 1,25-(OH)2D3 is mediated via protein kinase C- and protein kinase A-dependent pathways. Biomaterials 2001, 22, 731–741.
- Boyan, B.D.; Lohmann, C.H.; Sisk, M.A.; Liu, Y.; Sylvia, V.L.; Cochran, D.L.; Dean, D.D.; Schwartz, Z. Both cyclooxygenase-1 and cyclooxygenase-2 mediate osteoblast response to titanium surface roughness. J. Biomed. Mater. Res. 2001, 55, 350–359.
- Olivares-Navarrete, R.; Hyzy, S.L.; Hutton, D.L.; Dunn, G.R.; Appert, C.; Boyan, B.D.; Schwartz, Z. Role of non-canonical Wnt signaling in osteoblast maturation on microstructured titanium surfaces. Acta Biomater. 2011, 7, 2740–2750.
- Olivares-Navarrete, R.; Hyzy, S.; Pan, Q.; Dunn, G.; Williams, J.K.; Schwartz, Z.; Boyan, B.D. Osteoblast maturation on microtextured titanium involves paracrine regulation of bone morphogenetic signaling. J. Biomed. Mater. Res. A 2015, 103, 1721–1731.
- Olivares-Navarrete, R.; Hyzy, S.L.; Hutton, D.L.; Erdman, C.P.; Wieland, M.; Boyan, B.D.; Schwartz, Z. Direct and indirect effects of microstructured titanium substrates on the induction of mesenchymal stem cell differentiation towards the osteoblast lineage. Biomaterials 2010, 31, 2728–2735.
- Olivares-Navarrete, R.; Raz, P.; Zhao, G.; Chen, J.; Wieland, M.; Cochran, D.L.; Chaudhri, R.A.; Ornoy, A.; Boyan, B.D.; Schwartz, Z. Integrin alpha2beta1 plays a critical role in osteoblast response to micron-scale surface structure and surface energy of titanium substrates. PNAS 2008, 105, 15767–15772.
- Olivares-Navarrete, R.; Hyzy, S.; Wieland, M.; Boyan, B.D.; Schwartz, Z. The roles of Wnt signaling modulators Dickkopf-1 (Dkk1) and Dickkopf-2 (Dkk2) and cell maturation state in osteogenesis on microstructured titanium surfaces. Biomaterials 2010, 31, 2015–2024.
- Olivares-Navarrete, R.; Hyzy, S.L.; Park, J.H.; Dunn, G.R.; Haithcock, D.A.; Wasilewski, C.E.; Boyan, B.D.; Schwartz, Z. Mediation of osteogenic differentiation of human mesenchymal stem cells on titanium surfaces by a Wnt-integrin feedback loop. Biomaterials 2011, 32, 6399–6411.
- Galli, C.; Piemontese, M.; Lumetti, S.; Manfredi, E.; Macaluso, G.M.; Passeri, G. The Importance of Wnt Pathways for Bone Metabolism and Their Regulation by Implant Topography. Eur. Cells Mater. 2012, 24, 46–59.
- Wang, W.; Zhao, L.; Ma, Q.; Wang, Q.; Chu, P.K.; Zhang, Y. The role of the Wnt/beta-catenin pathway in the effect of implant topography on MG63 differentiation. Biomaterials 2012, 33, 7993–8002.
- Olivares-Navarrete, R.; Rodil, S.E.; Hyzy, S.L.; Dunn, G.R.; Almaguer-Flores, A.; Schwartz, Z.; Boyan, B.D. Role of integrin subunits in mesenchymal stem cell differentiation and osteoblast maturation on graphitic carbon-coated microstructured surfaces. Biomaterials 2015, 51, 69–79.
- Wang, W.; Zhao, L.; Wu, K.; Ma, Q.; Mei, S.; Chu, P.K.; Wang, Q.; Zhang, Y. The role of integrin-linked kinase/beta-catenin pathway in the enhanced MG63 differentiation by micro/nano-textured topography. Biomaterials 2013, 34, 631–640.
- Lüthen, F.; Lange, R.; Becker, P.; Rychly, J.; Beck, U.; Nebe, B. The influence of surface roughness of titanium on b1 and b3-integrin adhesion and the organization of fibronectin in human osteoblastic cells. Biomaterials 2005, 15, 2423–2440.
- Jang, T.S.; Jung, H.D.; Kim, S.; Moon, B.S.; Baek, J.; Park, C.; Song, J.; Kim, H.E. Multiscale porous titanium surfaces via a two-step etching process for improved mechanical and biological performance. Biomed. Mater. 2017, 12, 025008.
- Li, Y.; Qi, Y.; Gao, Q.; Niu, Q.; Shen, M.; Fu, Q.; Hu, K.; Kong, L. Effects of a micro/nano rough strontium-loaded surface on osseointegration. Int. J. Nanomed. 2015, 10, 4549–4563.
- Hasan, J.; Jain, S.; Chatterjee, K. Nanoscale Topography on Black Titanium Imparts Multi-biofunctional Properties for Orthopedic Applications. Sci. Rep. 2017, 7, 41118.
- Ren, B.; Wan, Y.; Liu, C.; Wang, H.; Yu, M.; Zhang, X.; Huang, Y. Improved osseointegration of 3D printed Ti-6Al-4V implant with a hierarchical micro/nano surface topography: An in vitro and in vivo study. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111505.
- Chen, X.; Chen, Y.; Shen, J.; Xu, J.; Zhu, L.; Gu, X.; He, F.; Wang, H. Positive modulation of osteogenesis on a titanium oxide surface incorporating strontium oxide: An in vitro and in vivo study. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 710–718.
- Zhang, Y.; Gong, H.; Sun, Y.; Huang, Y.; Fan, Y. Enhanced osteogenic differentiation of MC3T3-E1 cells on grid-topographic surface and evidence for involvement of YAP mediator. J. Biomed. Mater. Res. A 2016, 104, 1143–1152.
- De Luca, A.C.; Zink, M.; Weidt, A.; Mayr, S.G.; Markaki, A.E. Effect of microgrooved surface topography on osteoblast maturation and protein adsorption. J. Biomed. Mater. Res. A 2015, 103, 2689–2700.
- Pilia, M.; Guda, T.; Shiels, S.M.; Appleford, M.R. Influence of substrate curvature on osteoblast orientation and extracellular matrix deposition. J. Biol. Eng. 2013, 7, 23.
- Watari, S.; Hayashi, K.; Wood, J.A.; Russell, P.; Nealey, P.F.; Murphy, C.J.; Genetos, D.C. Modulation of osteogenic differentiation in hMSCs cells by submicron topographically-patterned ridges and grooves. Biomaterials 2012, 33, 128–136.
- Klymov, A.; te Riet, J.; Mulder, P.; Gardeniers, J.G.; Jansen, J.A.; Walboomers, X.F. Nanometer-grooved topography stimulates trabecular bone regeneration around a concave implant in a rat femoral medulla model. Nanomedicine 2016, 12, 2283–2290.
- Miyahara, K.; Watamoto, T.; Uto, Y.; Sawase, T. Effect of Macroscopic Grooves on Bone Formation and Osteoblastic Differentiation. Implant Dent. 2015, 24, 370–376.
- Dhawan, U.; Pan, H.A.; Shie, M.J.; Chu, Y.H.; Huang, G.S.; Chen, P.C.; Chen, W.L. The Spatiotemporal Control of Osteoblast Cell Growth, Behavior, and Function Dictated by Nanostructured Stainless Steel Artificial Microenvironments. Nanoscale Res. Lett. 2017, 12, 86.
- Ballo, A.; Agheli, H.; Lausmaa, J.; Thomsen, P.; Petronis, S. Nanostructured model implants for in vivo studies: Influence of well-defined nanotopography on de novo bone formation on titanium implants. Int. J. Nanomed. 2011, 6, 3415–3428.
- Karazisis, D.; Petronis, S.; Agheli, H.; Emanuelsson, L.; Norlindh, B.; Johansson, A.; Rasmusson, L.; Thomsen, P.; Omar, O. The influence of controlled surface nanotopography on the early biological events of osseointegration. Acta Biomater. 2017, 53, 559–571.
- Dalby, M.J.; McCloy, D.; Robertson, M.; Agheli, H.; Sutherland, D.; Affrossman, S.; Oreffo, R.O. Osteoprogenitor response to semi-ordered and random nanotopographies. Biomaterials 2006, 27, 2980–2987.
- Davison, M.J.; McMurray, R.J.; Smith, C.A.; Dalby, M.J.; Meek, R.D. Nanopit-induced osteoprogenitor cell differentiation: The effect of nanopit depth. J. Tissue Eng. 2016, 7.
- Hulshof, F.F.B.; Zhao, Y.; Vasilevich, A.; Beijer, N.R.M.; de Boer, M.; Papenburg, B.J.; van Blitterswijk, C.; Stamatialis, D.; de Boer, J. NanoTopoChip: High-throughput nanotopographical cell instruction. Acta Biomater. 2017.
- Curtis, A.S.G.; Wilkinson, C. Topographical control of cells. Biomaterials 1997, 18, 1573–1583.
- Weiss, P. Experiments on cell and axon orientation in vitro: The role of colloidal exudates in tissue organisation. J. Exp. Zool. 1945, 100, 353–386.
- Guillemette, M.D.; Cui, B.; Roy, E.; Gauvin, R.; Giasson, C.J.; Esch, M.B.; Carrier, P.; Deschambeault, A.; Dumoulin, M.; Toner, M.; et al. Surface topography induces 3D self-orientation of cells and extracellular matrix resulting in improved tissue function. Integr. Biol. 2009, 1, 196–204.
- Ishimoto, T.; Nakano, T.; Umakoshi, Y.; Yamamoto, M.; Tabata, Y. Degree of biological apatite c-axis orientation rather than bone mineral density controls mechanical function in bone regenerated using recombinant bone morphogenetic protein-2. J. Bone Miner. Res. 2013, 28, 1170–1179.
- Matsugaki, A.; Aramoto, G.; Ninomiya, T.; Sawada, H.; Hata, S.; Nakano, T. Abnormal arrangement of a collagen/apatite extracellular matrix orthogonal to osteoblast alignment is constructed by a nanoscale periodic surface structure. Biomaterials 2015, 37, 134–143.
- Nakanishi, Y.; Matsugaki, A.; Kawahara, K.; Ninomiya, T.; Sawada, H.; Nakano, T. Unique arrangement of bone matrix orthogonal to osteoblast alignment controlled by Tspan11-mediated focal adhesion assembly. Biomaterials 2019, 209, 103–110.
- Dalby, M.J.; Berry, C.C.; Riehle, M.O.; Sutherland, D.S.; Agheli, H.; Curtis, A.S. Attempted endocytosis of nano-environment produced by colloidal lithography by human fibroblasts. Exp. Cell Res. 2004, 295, 387–394.
- Ventre, M.; Causa, F.; Netti, P.A. Determinants of cell-material crosstalk at the interface: Towards engineering of cell instructive materials. J. R. Soc. Interface 2012, 9, 2017–2032.
- Nguyen, A.T.; Sathe, S.R.; Yim, E.K. From nano to micro: Topographical scale and its impact on cell adhesion, morphology and contact guidance. J. Phys. Condens. Matter 2016, 28, 183001.
- Gittens, R.A.; Olivares-Navarrete, R.; Schwartz, Z.; Boyan, B.D. Implant osseointegration and the role of microroughness and nanostructures: Lessons for spine implants. Acta Biomater. 2014, 10, 3363–3371.
- Anselme, K.; Ploux, L.; Ponche, A. Cell/material interfaces: Influence of surface chemistry and surface topography on cell adhesion. J. Adhes. Sci. Technol. 2010, 24, 831–852.
- Sartori, E.M.; Magro-Filho, O.; Silveira Mendonca, D.B.; Li, X.; Fu, J.; Mendonca, G. Modulation of Micro RNA Expression and Osteoblast Differentiation by Nanotopography. Int. J. Oral Maxillofac. Implants 2018, 33, 269–280.
- Rabel, K.; Kohal, R.J.; Steinberg, T.; Tomakidi, P.; Rolauffs, B.; Adolfsson, E.; Palmero, P.; Furderer, T.; Altmann, B. Controlling osteoblast morphology and proliferation via surface micro-topographies of implant biomaterials. Sci. Rep. 2020, 10, 12810.
- Lagonegro, P.; Trevisi, G.; Nasi, L.; Parisi, L.; Manfredi, E.; Lumetti, S.; Rossi, F.; Macaluso, G.M.; Salviati, G.; Galli, C. Osteoblasts preferentially adhere to peaks on micro-structured titanium. Dent. Mater. J. 2018, 37, 278–285.
- Migita, S.; Araki, K. Effect of nanometer scale surface roughness of titanium for osteoblast function. AIMS Bioeng. 2017, 4, 162–170.
- Ermis, M.; Antmen, E.; Hasirci, V. Micro and Nanofabrication methods to control cell-substrate interactions and cell behavior: A review from the tissue engineering perspective. Bioact. Mater. 2018, 3, 355–369.
- Di Cio, S.; Gautrot, J.E. Cell sensing of physical properties at the nanoscale: Mechanisms and control of cell adhesion and phenotype. Acta Biomater. 2016, 30, 26–48.
- Biggs, M.J.; Richards, R.G.; Gadegaard, N.; Wilkinson, C.D.; Dalby, M.J. Regulation of implant surface cell adhesion: Characterization and quantification of S-phase primary osteoblast adhesions on biomimetic nanoscale substrates. J. Orthop. Res. 2007, 25, 273–282.
- Lim, J.Y.; Dreiss, A.D.; Zhou, Z.; Hansen, J.C.; Siedlecki, C.A.; Hengstebeck, R.W.; Cheng, J.; Winograd, N.; Donahue, H.J. The regulation of integrin-mediated osteoblast focal adhesion and focal adhesion kinase expression by nanoscale topography. Biomaterials 2007, 28, 1787–1797.
- Seo, C.H.; Jeong, H.; Feng, Y.; Montagne, K.; Ushida, T.; Suzuki, Y.; Furukawa, K.S. Micropit surfaces designed for accelerating osteogenic differentiation of murine mesenchymal stem cells via enhancing focal adhesion and actin polymerization. Biomaterials 2014, 35, 2245–2252.
- Biggs, M.J.P.; Richards, R.G.; Gadegaard, N.; Wilkinson, C.D.W.; Dalby, M.J. The effects of nanoscale pits on primary human osteoblast adhesion formation and cellular spreading. J. Mater. Sci. Mater. Med. 2007, 18, 399–404.
- Biggs, M.J.; Richards, R.G.; McFarlane, S.; Wilkinson, C.D.; Oreffo, R.O.; Dalby, M.J. Adhesion formation of primary human osteoblasts and the functional response of mesenchymal stem cells to 330 nm deep microgrooves. J. R. Soc. Interface 2008, 5, 1231–1242.
- Biggs, M.J.; Richards, R.G.; Gadegaard, N.; Wilkinson, C.D.; Oreffo, R.O.; Dalby, M.J. The use of nanoscale topography to modulate the dynamics of adhesion formation in primary osteoblasts and ERK/MAPK signalling in STRO-1+ enriched skeletal stem cells. Biomaterials 2009, 30, 5094–5103.
- Cassidy, J.W.; Roberts, J.N.; Smith, C.A.; Robertson, M.; White, K.; Biggs, M.J.; Oreffo, R.O.; Dalby, M.J. Osteogenic lineage restriction by osteoprogenitors cultured on nanometric grooved surfaces: The role of focal adhesion maturation. Acta Biomater. 2014, 10, 651–660.
- Hamilton, D.W.; Brunette, D.M. The effect of substratum topography on osteoblast adhesion mediated signal transduction and phosphorylation. Biomaterials 2007, 28, 1806–1819.
- Lopes, H.B.; Freitas, G.P.; Elias, C.N.; Tye, C.; Stein, J.L.; Stein, G.S.; Lian, J.B.; Rosa, A.L.; Beloti, M.M. Participation of integrin beta3 in osteoblast differentiation induced by titanium with nano or microtopography. J. Biomed. Mater. Res. A 2019, 107, 1303–1313.
- Lopes, H.B.; Freitas, G.P.; Fantacini, D.M.C.; Picanco-Castro, V.; Covas, D.T.; Rosa, A.L.; Beloti, M.M. Titanium with nanotopography induces osteoblast differentiation through regulation of integrin alphaV. J. Cell. Biochem. 2019, 120, 16723–16732.
- Sonam, S.; Sathe, S.R.; Yim, E.K.; Sheetz, M.P.; Lim, C.T. Cell contractility arising from topography and shear flow determines human mesenchymal stem cell fate. Sci. Rep. 2016, 6, 20415.
- Zhang, T.; Lin, S.; Shao, X.; Zhang, Q.; Xue, C.; Zhang, S.; Lin, Y.; Zhu, B.; Cai, X. Effect of matrix stiffness on osteoblast functionalization. Cell Prolif. 2017, 50.
- Ge, C.; Xiao, G.; Jiang, D.; Franceschi, R.T. Critical role of the extracellular signal-regulated kinase-MAPK pathway in osteoblast differentiation and skeletal development. J. Cell Biol. 2007, 176, 709–718.
- Dalby, M.J.; Andar, A.; Nag, A.; Affrossman, S.; Tare, R.; McFarlane, S.; Oreffo, R.O. Genomic expression of mesenchymal stem cells to altered nanoscale topographies. J. R. Soc. Interface 2008, 5, 1055–1065.
- Wang, W.; Liu, Q.; Zhang, Y.M.; Zhao, L.Z. Involvement of ILK/ERK1/2 and ILK/p38 pathways in mediating the enhanced osteoblast differentiation by micro/nanotopography. Acta Biomater. 2014, 10, 3705–3715.
- Zhang, X.; Li, H.; Lin, C.; Ning, C.; Lin, K. Synergetic topography and chemistry cues guiding osteogenic differentiation in bone marrow stromal cells through ERK1/2 and p38 MAPK signaling pathway. Biomater. Sci. 2018, 6, 418–430.
- Rahman, M.S.; Akhtar, N.; Jamil, H.M.; Banik, R.S.; Asaduzzaman, S.M. TGF-beta/BMP signaling and other molecular events: Regulation of osteoblastogenesis and bone formation. Bone Res. 2015, 3, 15005.
- Castro-Raucci, L.M.S.; Francischini, M.S.; Teixeira, L.N.; Ferraz, E.P.; Lopes, H.B.; de Oliveira, P.T.; Hassan, M.Q.; Losa, A.L.; Beloti, M.M. Titanium With Nanotopography Induces Osteoblast Differentiation by Regulating Endogenous Bone Morphogenetic Protein Expression and Signaling Pathway. J. Cell. Biochem. 2016, 117, 1718–1726.
- Chakravorty, N.; Hamlet, S.; Jaiprakash, A.; Crawford, R.; Oloyede, A.; Alfarsi, M.; Xiao, Y.; Ivanovski, S. Pro-osteogenic topographical cues promote early activation of osteoprogenitor differentiation via enhanced TGFbeta, Wnt, and Notch signaling. Clin. Oral Implants Res. 2014, 25, 475–486.
- Souza, A.T.P.; Bezerra, B.L.S.; Oliveira, F.S.; Freitas, G.P.; Bighetti Trevisan, R.L.; Oliveira, P.T.; Rosa, A.L.; Beloti, M.M. Effect of bone morphogenetic protein 9 on osteoblast differentiation of cells grown on titanium with nanotopography. J. Cell. Biochem. 2018, 119, 8441–8449.
- Yang, J.; McNamara, L.E.; Gadegaard, N.; Alakpa, E.V.; Burgess, K.V.; Meek, R.M.; Dalby, M.J. Nanotopographical induction of osteogenesis through adhesion, bone morphogenic protein cosignaling, and regulation of microRNAs. ACS Nano 2014, 8, 9941–9953.
- Yavropoulou, M.P.; Yovos, J.G. The role of the Wnt signaling pathway in osteoblast commitment and differentiation. Hormones (Athens) 2007, 6, 279–294.
- Li, G.; Song, Y.; Shi, M.; Du, Y.; Wang, W.; Zhang, Y. Mechanisms of Cdc42-mediated rat MSC differentiation on micro/nano-textured topography. Acta Biomater. 2017, 49, 235–246.
- Wang, C.C.; Jamal, L.; Janes, K.A. Normal morphogenesis of epithelial tissues and progression of epithelial tumors. Wiley Interdiscip. Rev. Syst. Biol. Med. 2012, 4, 51–78.
- Li, L.; Yang, S.; Xu, L.; Li, Y.; Fu, Y.; Zhang, H.; Song, J. Nanotopography on titanium promotes osteogenesis via autophagy-mediated signaling between YAP and beta-catenin. Acta Biomater. 2019, 96, 674–685.
- McNamara, L.E.; Burchmore, R.; Riehle, M.O.; Herzyk, P.; Biggs, M.J.P.; Wilkinson, C.D.W.; Curtis, A.S.G.; Dalby, M.J. The role of microtopography in cellular mechanotransduction. Biomaterials 2012, 33, 2835–2847.
- Tsimbouri, P.M.; Murawski, K.; Hamilton, G.; Herzyk, P.; Oreffo, R.O.C.; Gadegaard, N.; Dalby, M.J. A genomics approach in determining nanotopographical effects on MSC phenotype. Biomaterials 2013, 34, 2177–2184.
- Pieuchot, L.; Marteau, J.; Guignandon, A.; dos Santos, T.; Brigaud, I.; Chauvy, P.F.; Cloatre, T.; Ponche, A.; Petithory, T.; Rougerie, P.; et al. Curvotaxis directs cell migration through cell-scale curvature landscapes. Nat. Commun. 2018, 9, 3995.
- Werner, M.; Blanquer, S.B.; Haimi, S.P.; Korus, G.; Dunlop, J.W.; Duda, G.N.; Grijpma, D.W.; Petersen, A. Surface Curvature Differentially Regulates Stem Cell Migration and Differentiation via Altered Attachment Morphology and Nuclear Deformation. Adv. Sci. (Weinh.) 2017, 4, 1600347.