Pain can be induced by tissue injuries, diseases and infections. The interactions between the peripheral nervous system (PNS) and immune system are primary actions in pain sensitizations. In response to stimuli, nociceptors release various mediators from their terminals that potently activate and recruit immune cells, whereas infiltrated immune cells further promote sensitization of nociceptors and the transition from acute to chronic pain by producing cytokines, chemokines, lipid mediators and growth factors. Immune cells not only play roles in pain production but also contribute to PNS repair and pain resolution by secreting anti-inflammatory or analgesic effectors.
- peripheral nervous system
- pain
- immune response
- inflammation
1. Introduction
Pain can be induced by tissue injury or different types of diseases that affect the somatosensory system, resulting in noxious (hyperalgesia) or non-noxious (allodynia) symptoms, which is an important defense mechanism to avoid harmful stimuli. Terminal nerves of somatosensory neurons (also known as nociceptors) innervate into the skin, cornea, internal organs, joints, bones, muscles, and deep visceral tissues, which are highly expressing a set of molecular sensors including transient receptor potential channel subtypes (TRPs), G protein coupled receptors (GPCRs) and sodium channel (Nav) [1,2][1][2]. Upon sensing noxious stimuli (e.g., mechanical, thermal and chemical), these nociceptors can quickly generate action potentials that are transmitted to the central nervous system (CNS) where the signals are processed. Nociceptor sensitization (or peripheral sensitization) at the site of the injury is therefore considered to be the primary cause of pain and the most appropriate targeting system for pain therapies [3,4][3][4].
Pain syndromes can be divided into acute and chronic stages. Acute pain plays a vital protective and adaptive role in warning the individual to avoid further injury and driving immune responses against infections or pathogens during healing. The inflammatory mediators produced by the immune system such as cytokines, lipid mediators, and growth factors directly activate nociceptive primary sensory neurons in the peripheral nervous system (PNS) evoking a pain response [4,5][4][5]. On the other hand, chronic pain is detrimental and arises from nerve damage caused by surgery, trauma, metabolic disorders (e.g., diabetic mellitus) or autoimmune diseases (e.g., multiple sclerosis) [6]. Chronic pain is a long-lasting syndrome and has substantial impacts on patients’ quality of life and high economic burdens on individuals and society [7]. Although alterations in the dorsal spinal cord and brain are one of the key mechanisms of chronic pain maintenance, peripheral sensitization is essential in the transition from the acute to the chronic stage [5,8][5][8]. Notably, emerging studies have revealed that bidirectional signaling between the immune and nervous systems contribute to the initiation and maintenance of chronic pain [2]. Altogether, our current knowledge of nociceptor–immune interactions have provided some molecular insights for developing better therapies for pathological inflammation-associated pain.
2. Peripheral Responses to Pain
Like the counterparts in the CNS, the PNS is also composed of neurons and glial cells, in which clusters of nociceptive sensory neurons are located in different ganglion in the trunk and the head that relay information about the environment to the CNS. The most common types of ganglion are the dorsal root ganglion (DRG) in the trunk, and others in the cranial including trigeminal and glossopharyngeal ganglia [9]. These sensory neurons (first-order primary afferent neurons) are classified to unmyelinated c-fibers and myelinated Aδ/β -fibers that transduce different types of pain signals, including mechanical, thermal, or chemical stimuli (Figure 1a). Free peripheral nerve endings function as receptive sites extend from neuronal cell bodies in the DRG or cranial nerve ganglion. Notably, their sensory neurons are pseudo-unipolar neurons that have one axon with two processes: one peripheral axonal branch innervates the tissues in the body to receive sensory information and the other axonal branch sends nerve impulses to excite second-order postsynaptic neurons in the dorsal horn of the spinal cord [3,5][3][5]. Subsequently, axons from second-order neurons project into thalamic nuclei in brain, where the third-order neurons transmit the pain signaling to the primary sensory cortex [10] (Figure 1b). The glial cells in the PNS mainly comprise Schwann cells and satellite glial cells (SGCs). The SGCs surround the somata of sensory neurons and usually consist of a single layer of cells connected to each other by gap junctions [11]. Schwann cells are the most abundant cell types in the PNS, which support axonal outgrowth by producing a variety of growth factors, such as nerve growth factor (NGF), glial cell line derived neurotrophic factor (GDNF), and brain-derived neurotrophic factor (BDNF) [12,13][12][13]. Schwann cells consist of two major phenotypes, myelinating Schwann cells and nonmyelinating Schwann cells [12]. Myelinating Schwann cells wrap larger axons in a 1:1 ratio to form the myelin sheath, nonmyelinating Schwann cells embed smaller axons, forming a remark bundle [13] (Figure 1c).
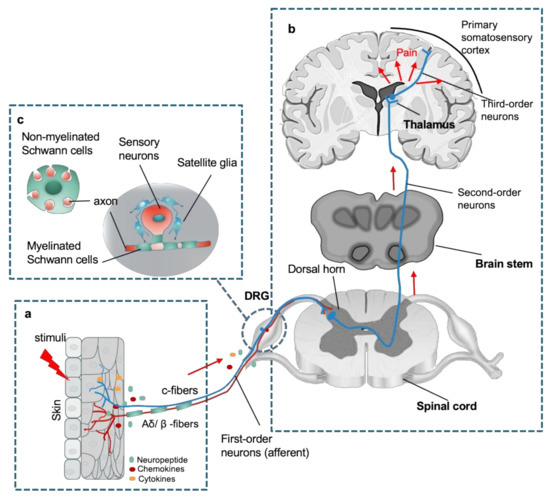
An overview of peripheral nervous system (PNS) in the sensory pathways leading from the skin to the brain. (
) The peripheral nerve endings comprise both unmyelinated c-fibers and myelinated Aδ/β -fibers in the skin that sense a stimulus, where chemicals such as inflammatory mediators and neuropeptides released from the injury site or the nerve endings activate the receptors and channels on the adjacent peripheral nerve terminals, subsequently resulting in initiating an action potential at the initial segment of the axon. (
) The axon of the peripheral sensory neuron (first order neuron) enters the spinal cord and contacts second-order neuron in the gray matter, where an action potential is generated at the initial segment of this neuron and travels up the sensory pathway to a region of the brainstem and thalamic nuclei. The sensory signal reaches the third-order neurons from the thalamus, and these project pain signaling to several cortical and subcortical regions (red arrows). (
) Schematic of organization of dorsal root ganglion (DRG). Sensory neuronal bodies are separated and wrapped satellite glial sheath. Myelinated Schwann cells envelop large diameter axons of sensory neurons, whereas nonmyelinated Schwann cells ensheath small diameter axons forming a remark bundle (upper left in (
)).
After noxious stimuli, peripheral neurons/nerves and glial cells undergo significant pathological changes before central properties that contribute to the pain initiation and development through their interaction with immune signals. Noteworthy, signaling pathways between primary sensory neurons, SGCs, Schwann cells and immune cells are highly intertwined. For example, activated Schwann cells mediate the breakdown of the blood–nerve barrier via the secretion of matrix metalloproteinase 9 (MMP-9), which promotes the recruitment and infiltration of immune cells (e.g., macrophages and T cells) from the vasculature to the injury sites. [14,15][14][15]. Sensory neurons also produce neuropeptides at their peripheral endings that not only serve as attractions to induce the invasion of circulating immune cells but also modulate the activity of innate and adaptive immune cells [2,16][2][16]. A dense cluster of immune cells produce pronociceptive mediators directly acting on peripheral nociceptors to promote sensitization of pain signaling and the recruitment of immune cells and vice versa. In addition, reactive SGCs and immune cells (e.g., macrophages) work cooperatively to promote peripheral sensitization by releasing proinflammatory cytokines such as IL-1β, IL-6, and TNF [17,18,19,20,21][17][18][19][20][21]. In contrast, SGCs and macrophages are also involved in the regeneration of DRG axons and remyelination of Schwann cells [21,22][21][22]. The four major heterogeneous immune cell types (macrophages and/or monocytes, mast cells, neutrophils, and T cells) resident in or infiltrating the PNS have specific functions in pain modulation and sensitization.
References
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284.
- Baral, P.; Udit, S.; Chiu, I.M. Pain and immunity: Implications for host defence. Nat. Rev. Immunol. 2019, 19, 433–447.
- Chung, G.; Jung, S.J.; Oh, S.B. Cellular and molecular mechanisms of dental nociception. J. Dent. Res. 2013, 92, 948–955.
- Slavin, K.V. Peripheral nerve stimulation for neuropathic pain. Neurotherapeutics 2008, 5, 100–106.
- Gold, M.S.; Gebhart, G.F. Nociceptor sensitization in pain pathogenesis. Nat. Med. 2010, 16, 1248–1257.
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B.; et al. Neuropathic pain. Nat. Rev. Dis. Primers 2017, 3, 17002.
- Inoue, S.; Taguchi, T.; Yamashita, T.; Nakamura, M.; Ushida, T. The prevalence and impact of chronic neuropathic pain on daily and social life: A nationwide study in a Japanese population. Eur. J. Pain 2017, 21, 727–737.
- Ji, R.R.; Chamessian, A.; Zhang, Y.Q. Pain regulation by non-neuronal cells and inflammation. Science 2016, 354, 572–577.
- Hossain, M.Z.; Unno, S.; Ando, H.; Masuda, Y.; Kitagawa, J. Neuron-Glia Crosstalk and Neuropathic Pain: Involvement in the Modulation of Motor Activity in the Orofacial Region. Int. J. Mol. Sci. 2017, 18, 2051.
- Grace, P.M.; Hutchinson, M.R.; Maier, S.F.; Watkins, L.R. Pathological pain and the neuroimmune interface. Nat. Rev. Immunol. 2014, 14, 217–231.
- Rozanski, G.M.; Li, Q.; Stanley, E.F. Transglial transmission at the dorsal root ganglion sandwich synapse: Glial cell to postsynaptic neuron communication. Eur. J. Neurosci. 2013, 37, 1221–1228.
- Kidd, G.J.; Ohno, N.; Trapp, B.D. Biology of Schwann cells. Handb. Clin. Neurol. 2013, 115, 55–79.
- Wei, Z.; Fei, Y.; Su, W.; Chen, G. Emerging Role of Schwann Cells in Neuropathic Pain: Receptors, Glial Mediators and Myelination. Front. Cell Neurosci. 2019, 13, 116.
- Shubayev, V.I.; Angert, M.; Dolkas, J.; Campana, W.M.; Palenscar, K.; Myers, R.R. TNFalpha-induced MMP-9 promotes macrophage recruitment into injured peripheral nerve. Mol. Cell Neurosci. 2006, 31, 407–415.
- Shubayev, V.I.; Myers, R.R. Upregulation and interaction of TNFalpha and gelatinases A and B in painful peripheral nerve injury. Brain Res. 2000, 855, 83–89.
- Chiu, I.M.; von Hehn, C.A.; Woolf, C.J. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat. Neurosci. 2012, 15, 1063–1067.
- Dubovy, P.; Klusakova, I.; Svizenska, I.; Brazda, V. Satellite glial cells express IL-6 and corresponding signal-transducing receptors in the dorsal root ganglia of rat neuropathic pain model. Neuron. Glia Biol. 2010, 6, 73–83.
- Huang, L.Y.; Gu, Y.; Chen, Y. Communication between neuronal somata and satellite glial cells in sensory ganglia. Glia 2013, 61, 1571–1581.
- Afroz, S.; Arakaki, R.; Iwasa, T.; Oshima, M.; Hosoki, M.; Inoue, M.; Baba, O.; Okayama, Y.; Matsuka, Y. CGRP Induces Differential Regulation of Cytokines from Satellite Glial Cells in Trigeminal Ganglia and Orofacial Nociception. Int. J. Mol. Sci. 2019, 20, 711.
- Takeda, M.; Takahashi, M.; Matsumoto, S. Contribution of the activation of satellite glia in sensory ganglia to pathological pain. Neurosci. Biobehav. Rev. 2009, 33, 784–792.
- Hanani, M.; Spray, D.C. Emerging importance of satellite glia in nervous system function and dysfunction. Nat. Rev. Neurosci. 2020, 21, 485–498.
- Carlin, D.; Halevi, A.E.; Ewan, E.E.; Moore, A.M.; Cavalli, V. Nociceptor Deletion of Tsc2 Enhances Axon Regeneration by Inducing a Conditioning Injury Response in Dorsal Root Ganglia. eNeuro 2019, 6.
