Flavonols are ones of the most common phytochemicals found in diets rich in fruit and vegetables. Research suggests that molecular functions of flavonoids may bring a number of health benefits to people, including the following: decrease inflammation, change disease activity, and alleviate resistance to antibiotics as well as chemotherapeutics.
- flavonols
- head and neck cancer prevention
- molecular mechanism
1. Introduction
Morbidity of neoplastic diseases and cancer mortality increase at an accelerated pace in both developed and developing countries. New Globocan 2020 data regarding cancer and including 185 countries indicated over 19 million new cancer cases and over 9.8 million deaths associated with cancer. In spite of medical advancements in diagnostics and treatment of neoplastic diseases, cancers remain the most common cause of death around the world [1]. Limited funding for prophylactic measures (including prophylaxis against neoplastic diseases) due to low budget is both a Europe-wide and worldwide problem. Successful strategies for dealing with threats such as cancer are needed. Flavonoids are well-known plant products and, according to numerous reports, possess biological activities directed against many diseases, such as malignant neoplasms, including head and neck cancer.
Head and neck squamous cell carcinomas (HNSCC) include, first and foremost, cancers that develop in the upper part of the respiratory tract and the gastrointestinal tract. Global statistics indicate that HNSCC occur worldwide with the frequency of 500,000 cases per year. In the recent years, a considerable increase in morbidity and mortality caused by malignant neoplasms, including head and neck cancer, has been observed. All epithelial tumors constitute a great challenge in clinical practice; however, it is the anatomy of tumors in the area of head and neck that makes them especially difficult to treat. Even though novel methods have improved locoregional control in patients with advanced HNSCC, locoregional, and distant recurrences are common and almost always lead to death.
2. Flavonols
Structure of Flavonols
Flavonols are a major class of the family of flavonoids. Flavonoids are 2 phenyl benzo-γ-pyrone derivatives. A common part in the chemical structure of all flavonoids is a 15-carbon skeleton based on a flavan structure (C6-C3-C6) consisting of two aromatic rings (A and B) linked by a heterocyclic pyrane or pyrone ring (C). A wide diversity of flavonoids stems from the fact that carbon atoms of A, B and C rings may undergo hydroxylation, methoxylation and glycosidation by means of mono- and oligosaccharides, as well as acylation at different positions. Biogenesis of flavonoids is a complex process. They are created from precursors of primary metabolism during a transformation creating two pathways, as shown in Figure 1. The first one is a malonic acid pathway, in which malonyl coenzyme A is created from acetyl coenzyme A produced through glycolysis; three molecules of malonyl-CoA obtained from glucose transformation are the source of the aromatic ring A. The second one is a shikimic acid pathway, in which erythrose-4-phosphate derived from the pentose phosphate pathway and phosphoenolpyruvate created via glycolysis are transformed into shikimic acid, and then, into aromatic amino acids: phenylalanine and tyrosine. During subsequent transformations, both amino acids undergo deamination, and consequently, form cinnamic acid. Condensation of ring A and ring B results in creation of chalcone, which undergoes cyclization with isomerase, and a flavanone is produced—a starting compound for synthesis of the remaining groups of flavonoids [2][3][3,4].
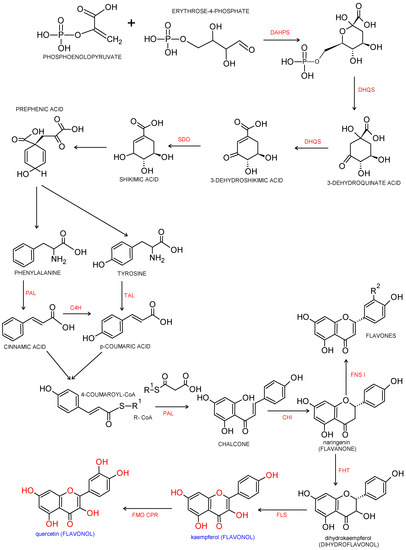
Figure 1. Biosynthesis pathway of phenylpropanoids and flavonols. Biosynthesis of flavonols from p-coumaroyl-CoA and malonyl-CoA. Enzymatic reaction catalyzed by DAHPS: 3-deoxy-D-arabino-heptulosonate-7-phosphate-synthase; DHQS: 3-dexydroquinate synthase; SDO: shikimate dehydrogenase; PAL: phenylalanine ammonia-lyase; TAL: tyrosine ammonia lyase; C4H: cinnamate 4-hydroxylase; CHI: chalcone isomerase, FHT: flavanone 3β-hydroxylase; FNS Ι: flavone synthase Ι; FLS: flavonol synthase; FMO: flavonoid 3′-monooxygenase; CPR: cytochrome P450reductase [4][5][6][8,9,10].
All naturally found flavonoids have three hydroxyl groups: two found in ring A at position 5 and 7, and one in ring B at position 3. Diverse positions of substituents in a flavonoid molecule give it different chemical and physical properties, which translates into an individual metabolism of a given compound and its biological activity. Flavonoids, except for isoflavones, have their aromatic ring attached to pyrone C2, whereas in isoflavones it is attached to C3 [2][3][7][8][9][3,4,5,6,7].
Chemically, flavonols differ from many other flavonoids, because they have a double bond between position 2 and position 3 as well as oxygen (a ketone group) at position 4 of ring C similarly to flavones, from which they yet differ due to the presence of hydroxyl group at position 3 (FigureFigure 2 2 and Figure 3).

Figure 2. Basic skeleton or structure of flavonols.
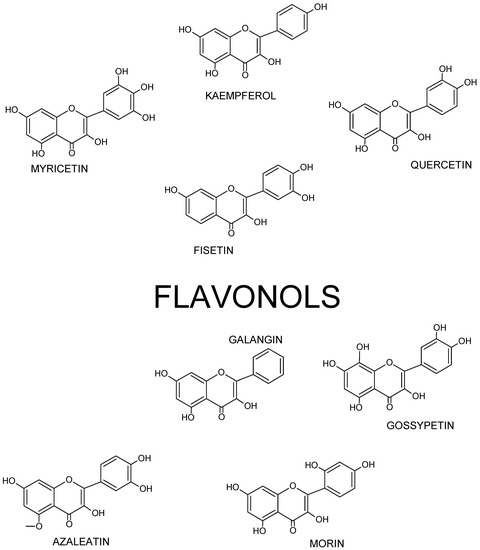
Figure 3. Structures of selected flavonols.
Thus, flavonol’s skeleton is 3-hydroxyflavone. 3′ hydroxyl group may attach sugar, i.e., it might be glycosylated. It was shown that three structural groups are important for biological activity, including anti-oxidative and radical-scavenging activity of flavonoids: (1) o-dihydroxyl group in ring B; (2) double bond at 2,3 attached to 4-oxy group in ring C; and (3) hydroxyl groups at positions C3 and C5. Moreover, a 3′,4′-o-dihydroxyl group is an indicator of a considerable antioxidant activity of flavonoids, especially those that do not belong to the flavonol subclass. Ring B catechol group is critical for high antioxidant activity. A higher antioxidant activity of flavonoids with o-dihydroxyl group in ring B is attributed to their greater radical stability via an increased delocalization of electrons and intramolecular hydrogen bonds between 3′- and 4′-hydroxyl groups. Additionally, 5′-hydroxyl group in ring B, as seen in myricetin and azaleatin, decreases the antioxidant activity, which might be caused by a prooxidant activity introduced by a pyrogallol group. [10][11][12][11,12,13].
3. Anticancer Potential of Flavonols
3.1. Head and Neck Cancer
Epidemiological data indicate that neoplasms regarding head and neck organs are more common in men (about 66–95% of cases), and the morbidity rate with regard to sex differs depending on anatomical site and changes with an increasing percentage of smoking women. Currently, the rate is 3:1 for oral cavity and oropharyngeal cancers. Prevalence of head and neck cancers increases with age, especially after the age of 45, and the peak for incidence is observed between the age of 50 and 70; however, these cancers also develop in younger individuals.
Their prevalence increases in poorly developed countries and in countries, in which resources are limited. These tumors are regarded as an important issue for public health in developed and developing countries. They are an important reason behind death of people due to their ability to disrupt basic life functions, such as breathing, speaking, hearing, seeing, smelling, or swallowing.
Neoplasms of this type are considered malignant; they develop on the mucous membrane of the air and digestive tracts and, thus, may be called cancers. They be appear in the following among other sites: the oral cavity, salivary glands, nasal cavity, paranasal sinuses, nasopharynx, hypopharynx and oropharynx, thyroid, larynx, ear, and lip. Due to high probability of metastases to neck lymph nodes, and in most cases a small risk of developing distal metastases (apart from the thyroid cancer), they are classified as locally malignant. Moreover, they are believed to be a group of highly heterogenic malignant tumors because their responsiveness to treatment and clinical course are conditioned to a large extent by their site.
Squamous cell carcinoma (SCC) is considered to be the most common histological type as it constitutes over 90% of cancers in those areas. In most cases, these are cancers found within the oral cavity, including the tongue and larynx (about 60–70%) [13][14][15][16][17][14,15,16,17,18]. Over 95% of cases are SCCs that are either well or moderately differentiated. Adenocarcinomas originating in small salivary glands are relatively rare.
In 2020, which is the last year with available data for morbidity, in worldwide there reported over 930,000 head and neck cancers, that is presented in Table 1.
Table 1. Estimated number of incident cases and mortality factor in Europe (EURO) and Pan American (PAHO) caused by different anatomical types of tumors in the head and neck region, acc. to WHO [1][18][1,19].
| Incidence | Mortalilty | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Worldwide | WHO Europe (EURO) | WHO Americas (PAHO) | Worldwide | WHO Europe (EURO) | WHO Americas (PAHO) | |||||||
| ENIC | ECI | ENIC | ECI | ENIC | ECI | END | ECM | END | ECM | END | ECM | |
| Lip, oral cavity | 377,713 | 4.8 | 69,856 | 7.5 | 45,357 | 4.4 | 177,757 | 2.3 | 26,530 | 2.8 | 12,533 | 1.2 |
| Larynx | 184,615 | 2.4 | 45,790 | 4.9 | 29,685 | 2.9 | 99,840 | 1.3 | 22,224 | 2.4 | 14,434 | 1.4 |
| Nasopharynx | 133,354 | 1.7 | 6820 | 0.73 | 4222 | 0.41 | 80,008 | 1 | 3537 | 0.38 | 2247 | 0.22 |
| Orophargynx | 98,412 | 1.3 | 30,061 | 3.2 | 22,910 | 2.2 | 48,143 | 0.62 | 13,664 | 1.5 | 8576 | 0.84 |
| Hypopharynx | 84,252 | 1.1 | 19,730 | 2.1 | 4892 | 0.48 | 38,599 | 0.5 | 9721 | 1 | 1740 | 0.17 |
| Salivary glands | 53,593 | 0.69 | 11,027 | 1.2 | 9791 | 0.96 | 22,778 | 0.29 | 4581 | 0.49 | 2399 | 0.23 |
Estimated crude incidence rate-ECI; Estimated crude mortality rate-ECM; Estimated number of incident cases-ENIC; Estimated number of deaths-END.
