Cancer is a major burden of disease globally. Each year, tens of millions of people are diagnosed with cancer worldwide, and more than half of the patients eventually die from it. Significant advances have been noticed in cancer treatment, but the mortality and incidence rates of cancers are still high. Thus, there is a growing research interest in developing more effective and less toxic cancer treatment approaches. Curcumin (CUR), the major active component of turmeric (Curcuma longa L.), has gained great research interest as an antioxidant, anticancer, and anti-inflammatory agent. This natural compound shows its anticancer effect through several pathways including interfering with multiple cellular mechanisms and inhibiting/inducing the generation of multiple cytokines, enzymes, or growth factors including IκB kinase β (IκKβ), tumor necrosis factor-alpha (TNF-α), signal transducer, and activator of transcription 3 (STAT3), cyclooxygenase II (COX-2), protein kinase D1 (PKD1), nuclear factor-kappa B (NF-κB), epidermal growth factor, and mitogen-activated protein kinase (MAPK). Interestingly, the anticancer activity of CUR has been limited primarily due to its poor water solubility, which can lead to low chemical stability, low oral bioavailability, and low cellular uptake. Delivering drugs at a controlled rate, slow delivery, and targeted delivery are other very attractive methods and have been pursued vigorously.
- Curcuma longa
- curcumin
- anticancer
- mechanism of action
- cellular mechanisms
Note: The following contents are extract from your paper. The entry will be online only after author check and submit it.
1. Introduction
Curcuma longa (C. longa)
Erythroxylumprevillei
Cephalotaxus
Betula alba
Catharanthusroseus
Taxusbrevifolia [2][4]. Furthermore, it has been demonstrated that numerous plant species exhibit anti-cancer properties and there is a growing interest regarding these plants, particularly in developing countries [5][6][7][8][9][10].
C. longa
21
20
6 [12]. CUR has shown its activity against multiple chronic diseases including neurodegenerative disorders, obesity, liver disease, metabolic syndrome, arthritis, inflammation, and multiple cancers [13][14]. Indeed, CUR is a highly effective candidate in cancer treatment as a single-drug therapy or in combination with other therapeutic agents.This natural compound also has the capacity to influence various molecular targets and signaling mechanisms that are linked with various cancers [15][16].
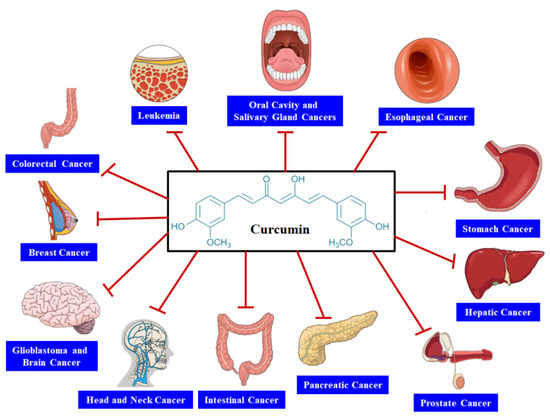
In this article, we have summarized CUR’s anticancer activity against several cancers such as gastrointestinal, brain, head and neck, pancreatic, prostate, breast, and colorectal cancers. Furthermore, we also focused on the findings obtained from several experimental and clinical studies regarding the anticancer action of CUR in animal models, human subjects, and cancer cell lines.
2. Mechanism of Action of Curcumin as an Anticancer Agent
An imbalance between cell death and cell proliferation is regarded as one of the major causal factors of cancer [17]. Uncontrolled cell proliferation is likely to occur if the cells skip death, which can lead to various types of cancers [18]. The intrinsic and extrinsic pathways are responsible for generating apoptotic signals. It has been found that the intrinsic pathway plays a role via inducing the mitochondrial membrane to suppress the expressions of B-cell lymphoma-extra large and B-cell lymphoma 2 (Bcl-2) [19]. CUR has the ability to disrupt the balance of mitochondrial membrane potential, which can result in increased Bcl-xL suppression [20]. On the other hand, the extrinsic apoptotic pathway functions via inducing the tumor necrosis factor (TNF)-associated apoptosis and elevating the death receptors (DRs) on cells. In this pathway, CUR plays a role via upregulating DR4 and DR expression [21][22][23]. It has been revealed by in vitro studies that CUR and its derivatives can excellently stimulate apoptosis in various cell lines through downregulating or suppressing intracellular transcription factors. These transcription factors include matrix metalloproteinase-9 (MMP-9), signal transducer and activator of transcription 3 (STAT3), cyclooxygenase II (COX-2), activator protein 1 (AP-1), nuclear factor-kappa B (NF-κB), and nitric oxide synthase [24][25]. CUR can also exert its anticancer effect via reducing lactate production and uptake of glucose in cancer cells through pyruvate kinase M2 (PKM2)downregulation. PKM2 suppression was found to be attained by inhibiting the mammalian target of rapamycin-hypoxia-inducible factor 1α (mTOR-HIF1α) [26]. Multiple analyses have examined the capacity of CUR and derivatives of CUR to inhibit several types of cancers through interacting with various molecular targets (
2. Mechanism of Action of Curcumin as an Anticancer Agent
In CL-5 xenograft tumors, CUR can trigger apoptosis and caused downregulation of cyclin D1, c-Met, Akt, and epidermal growth factor receptor (EGFR) [27]. Furthermore, CUR suppressed metastasis and lung cell invasion via upregulating the expression of HLJ1 in cancer cells [28]. Along with the activity of CUR on nuclear factor-κB (NF-κB) and STAT3 signaling cascades, CUR also suppressed cell cycle arrest and cell proliferation and induced apoptosis by modulating other transcription factors including PPAR-α, Hif-1, Notch-1, β-catenin, p53, Erg-1, and AP-1 [29]. It has been confirmed that CUR suppressed the phosphorylation of focal adhesion kinase (FAK) and increased the expression of multiple extracellular matrix (ECM) components, which further contribute to metastasis and invasion. In a concentration-dependent manner, CUR also increased cell adhesion via inducing various ECM components including fibronectin, laminin, collagen IX, collagen IV, collagen III, and collagen I. Collectively, these findings have indicated that CUR inhibits FAK action via suppression of its phosphorylation sites and triggers ECM components to improve cell adhesion, which can eventually prevent cell migration and detachment of cancer cells. It was reported that suppression of FAK expression resulted in elevated cell adhesion, which eventually playeda role in the anti-invasive activity of CUR [30]. In colorectal cancer cells, CUR decreased the expression of CD24 in a dose-dependent manner. In addition, expression of E-cadherin was elevated by CUR and played a role as a suppressor of epithelial-mesenchymal transition. In colorectal cancer cells, CUR may exhibit its action against metastasis by downregulating CD24, FAK, and Sp-1, and upregulating the expression of E-cadherin [30]. In a study, Zhou et al. [30] assessed the activity of 11 CUR-associated compounds (comprising a benzyl piperidone moiety) in various cancer cell lines. Furthermore, they observed that some of these compounds decreased the level of the phospho-extracellular signal-regulated kinase (Erk)1/2 and phospho-Akt [30]. It has been reported that autophagy and ER stress might have a significant contribution in the case of apoptosis, which is triggered via the CUR analogue B19 in hepatocellular carcinoma cells and the epithelial ovarian tumor cell line, and that suppression of autophagy may elevate CUR analogue-triggered apoptosis via stimulating severe ER stress. In ovarian cancer cell lines, this CUR analogue might also induce apoptosis, autophagy, and ER stress in vitro [31][32]. It was confirmed that autophagy may play role in programmed cell death type II and might effectively inhibit the growth of malignant glioma cells after treatment with CUR [33].
3. Bioavailability of Curcumin
CUR is safer to use and it has great potential as an anticancer agent. However, its major drawback is its poor oral bioavailability, which takes place because of its substantial first-pass effect and low aqueous solubility [34][35][36][37][38][39]. At tumor sites, increased permeability and retention action of nanomaterials might ameliorate the buildup of chemotherapeutic agents. In this regard, for instance, micelles, dendrimers, carbon nanotubes, and liposomes, have been utilized as carriers for cisplatin, paclitaxel, doxorubicin, and SN38 in order to decrease adverse effects and improve concentrations of drugs in tumors [39][40][41][42][43][44]. Increased solubility of chemotherapeutic drugs is another benefit of utilizing nanomaterials as drug carriers. There is a growing interest in self-assembling peptide nanofibers due to their easy modification, good biocompatibility, and design flexibility by a “bottom-up” technique [45][46]. These nanofibers have been extensively utilized in several cell cultures and also in drug delivery systems to decrease adverse effects, ameliorate buildup at the tumor site, and improve the solubility of a hydrophobic drug [47]. The enhanced anticancer activity has been observed with the nanofiber-encapsulated antitumor agents including ellipticine, camptothecin, and paclitaxel [48][49][50]. Several studies have confirmed that as drug carriers, the 2-dimensional structure of peptide nanofibers is much better compared to the 3-dimensional structure of nanoparticles. In a study, Wagh et al. [51] showed that peptide-based nanofibershada rapid elimination rate, improved tumor targeting within a shorter period, and better biocompatibility compared to carbon rods, and spherical nanomaterials (selenium and cadmium quantum dots, poly(lactic-co-glycolic acid) or PLGA, cadmium, gold, and polystyrene).
CUR has been widely studied and several synthetic analogues of CUR have been generated and analyzed for potential therapeutic effects [52][53][54][55][56][57][58][59]. Some of these analogues showed excellent actions in several cancer animal models and cell lines. It has been revealed by studies that CUR-associated compounds containing benzyl piperidone possess increased biological effects and absorption [60][61]. In addition, studies have also confirmed effective anticancer activities of CUR analogues [62][63][64][65]. The introduction of CUR into nano-formulations to increase water-solubility has outstandingly transformed its bioavailability. Moreover, nano-formulations have enhanced the transport and improved in vitro CUR levels in the cell, whereas their extended-release formulas as well as their increased compatibility appear to be excellent for their in vivo activities [66][67][68].
