Anthocyanins extracted from black carrots have received increased interest as natural colorants in recent years. The reason is mainly their high content of acylated anthocyanins that stabilizes the color and thereby increases the shelf-life of products colored with black carrot anthocyanins. Still, the main type of anthocyanins synthesized in all black carrot cultivars is cyanidin limiting their use as colorants due to the narrow color variation. Additionally, in order to be competitive against synthetic colors, a higher percentage of acylated anthocyanins and an increased anthocyanin content in black carrots are needed. However, along with the increased interest in black carrots there has also been an interest in identifying the structural and regulatory genes associated with anthocyanin biosynthesis in black carrots. Thus, huge progress in the identification of genes involved in anthocyanin biosynthesis has recently been achieved. Given this information it is now possible to attempt to modulate anthocyanin compositions in black carrots through genetic modifications.
1. Introduction
The anthocyanins have received increased interest as natural colorants for application in the food and beverage industry in recent years [1][2]. Although artificial food colorants are extensively used due to high stability and low costs, artificial colorants are under suspicion of being involved in hyperactivity of children and allergenicity [3][4][5]. Thus, there is an increasing demand from consumers for the use of natural colorants and this global trend is expected to increase [6].
The anthocyanins have received increased interest as natural colorants for application in the food and beverage industry in recent years [1,2]. Although artificial food colorants are extensively used due to high stability and low costs, artificial colorants are under suspicion of being involved in hyperactivity of children and allergenicity [3,4,5]. Thus, there is an increasing demand from consumers for the use of natural colorants and this global trend is expected to increase [6].
Anthocyanins are a group of colored water-soluble pigments found in plants, especially in fruits, flowers and tubers. Anthocyanins are glycosides and acylglycosides of anthocyanidins. Anthocyanidins are unstable in the cytosol and immediately after synthesis undergo
O
-glycosylation by formation of a glycosidic bond between the C
3
position of the C-ring and a sugar moiety resulting in formation of 3-
O
-monoglycoside anthocyanins (
). Furthermore, the sugar residues are sometimes acylated with aromatic or aliphatic acids at the C
6”
position of the sugar moiety [7]. The five major anthocyanidins synthesized in plants are pelargonidin, cyanidin, delphinidin, the O
-methylated derivate of cyanidin called peonidin, and the two
O
-methylated derivates of delphinidin called petunidin and malvidin (
). The color of anthocyanins is dependent on the type of anthocyanin pigment and the pH [5][8][9]. In nature, pelargonidin appears orange to red, cyanidin appears reddish-purple, peonidin appears magenta, delphinidin appears blue-reddish, petunidin appears dark red to purple, and malvidin appears purple in color [9].
). The color of anthocyanins is dependent on the type of anthocyanin pigment and the pH [5,8,9]. In nature, pelargonidin appears orange to red, cyanidin appears reddish-purple, peonidin appears magenta, delphinidin appears blue-reddish, petunidin appears dark red to purple, and malvidin appears purple in color [9].
Figure 1.
Basic structure of the six most common anthocyanidins.
As opposed to artificial food colorants, anthocyanins have low to no adverse effects. On the contrary, anthocyanins have been found to have health benefits because of their free radical scavenging, antioxidant, anticancer, and antimicrobial activity [9][10]. Still, the use of anthocyanins as natural colorants is often limited by their low stability, which can result in color loss or hue alterations. The stability is primarily dependent on the pH, temperature, light, and the degree of copigmentation and acylation [5][6][11][12][13]. First of all, the color of the anthocyanins is very dependent on the pH because the molecular structure has an ionic nature [9]. They exist in four pH-dependent forms i.e., as flavylium cation at pH 1–2 where some anthocyanin types appear in the reddish hue, as carbinol pseudo-base at pH 4-5 where they are colorless, as quinoidal base at pH 6–6.5 where they are bluish and as chalcone at pH 7 where they are pale yellow [14]. However, anthocyanin pigments form noncovalent complexes with other flavonoids (copigments) such as flavones and flavonols that stabilize the color [15]. This phenomenon is called copigmentation. The copigmentation complex is, however, more stable when the anthocyanin pigments are acylated as the acylated pigments forms more stable complexes when they are linked through the sugar residue by aromatic and/or aliphatic phenolic acyl moieties [16]. Therefore, acylated anthocyanins have improved color stability in the 4–5 pH range and retains the color in the mildly acidic pH environment of many food products as compared to nonacylated anthocyanins, which are nearly colorless at this pH range [6][16]. Acylated anthocyanins can also withstand degradation at higher temperatures and at longer light exposures [5][17]. As a result, food added acylated anthocyanin colorants have a longer shelf-life [18][19].
As opposed to artificial food colorants, anthocyanins have low to no adverse effects. On the contrary, anthocyanins have been found to have health benefits because of their free radical scavenging, antioxidant, anticancer, and antimicrobial activity [9,10]. Still, the use of anthocyanins as natural colorants is often limited by their low stability, which can result in color loss or hue alterations. The stability is primarily dependent on the pH, temperature, light, and the degree of copigmentation and acylation [5,6,11,12,13]. First of all, the color of the anthocyanins is very dependent on the pH because the molecular structure has an ionic nature [9]. They exist in four pH-dependent forms i.e., as flavylium cation at pH 1–2 where some anthocyanin types appear in the reddish hue, as carbinol pseudo-base at pH 4-5 where they are colorless, as quinoidal base at pH 6–6.5 where they are bluish and as chalcone at pH 7 where they are pale yellow [14]. However, anthocyanin pigments form noncovalent complexes with other flavonoids (copigments) such as flavones and flavonols that stabilize the color [15]. This phenomenon is called copigmentation. The copigmentation complex is, however, more stable when the anthocyanin pigments are acylated as the acylated pigments forms more stable complexes when they are linked through the sugar residue by aromatic and/or aliphatic phenolic acyl moieties [16]. Therefore, acylated anthocyanins have improved color stability in the 4–5 pH range and retains the color in the mildly acidic pH environment of many food products as compared to nonacylated anthocyanins, which are nearly colorless at this pH range [6,16]. Acylated anthocyanins can also withstand degradation at higher temperatures and at longer light exposures [5,17]. As a result, food added acylated anthocyanin colorants have a longer shelf-life [18,19].
Anthocyanins from black carrots (
Daucus carota
ssp.
sativus
var.
atrorubens Alef.) have some major advantages over anthocyanin extractions from fruits and other vegetables. Black carrot taproots have a high content of anthocyanins that can reach as high as 190 mg/100 g of fresh weight in some cultivars [20] and they also have a high degree of mono-acylated anthocyanins increasing their color stability [5][12][17][18]. However, anthocyanins from black carrots also have some limitations for the use as natural colorants. In black carrot the absolute major anthocyanin produced is cyanidin, although peonidin, pelargonidin and delphinidin have been found in small amounts in some cultivars [21][22][23][24]. Thus, anthocyanins from black carrots are today mainly used to produce colorants in the red hue. An extended anthocyanin color palette is, however, needed to fulfill color requirements for different foods e.g., beverages, dairy products and snacks. Additionally, a higher percentage of acylated anthocyanins would be desirable to increase the color stability. There is also a need for an increased anthocyanin content in black carrots in order to be competitive against synthetic colors. Industry estimation of the present production cost shows that the anthocyanin content must be increased at least 3 times in black carrots in order to be competitive against synthetic colors [25].
Alef.) have some major advantages over anthocyanin extractions from fruits and other vegetables. Black carrot taproots have a high content of anthocyanins that can reach as high as 190 mg/100 g of fresh weight in some cultivars [20] and they also have a high degree of mono-acylated anthocyanins increasing their color stability [5,12,17,18]. However, anthocyanins from black carrots also have some limitations for the use as natural colorants. In black carrot the absolute major anthocyanin produced is cyanidin, although peonidin, pelargonidin and delphinidin have been found in small amounts in some cultivars [21,22,23,24]. Thus, anthocyanins from black carrots are today mainly used to produce colorants in the red hue. An extended anthocyanin color palette is, however, needed to fulfill color requirements for different foods e.g., beverages, dairy products and snacks. Additionally, a higher percentage of acylated anthocyanins would be desirable to increase the color stability. There is also a need for an increased anthocyanin content in black carrots in order to be competitive against synthetic colors. Industry estimation of the present production cost shows that the anthocyanin content must be increased at least 3 times in black carrots in order to be competitive against synthetic colors [25].
Since the interest in cultivation of black carrots for production of anthocyanins has become increasingly high, there has also been an interest in identifying the structural and regulatory genes associated with anthocyanin biosynthesis in black carrots. Along with the publication of the carrot genome sequence by Xu et al. [26] and the high-quality sequence also assembled at the chromosome level by Iorizzo et al. [27], huge progress in the identification of genes involved in anthocyanin biosynthesis and genes involved in the secondary modifications i.e., glycosylation and acylation has been made [28]. Given this information it is now possible to attempt to modulate anthocyanin compositions in black carrots through genetic modifications.
2. Genes Responsible for Anthocyanin Biosynthesis in Black Carrots
Since the interest of black carrots for producing anthocyanin pigments has become increasingly high, there has also been an interest in identifying the structural and regulatory genes associated with anthocyanin biosynthesis in black carrots. In this review we will only include and refer to carrot anthocyanin structural and regulatory genes included in the recent review by Iorizzo et al. [28]. Here they integrated the structural genes from data of eight independent studies [29][30][31][32][33][34][35][36] and the regulatory genes from data of six independent studies [29][30][33][35][36][37]. The carrot genes included in this study are shown in the
Since the interest of black carrots for producing anthocyanin pigments has become increasingly high, there has also been an interest in identifying the structural and regulatory genes associated with anthocyanin biosynthesis in black carrots. In this review we will only include and refer to carrot anthocyanin structural and regulatory genes included in the recent review by Iorizzo et al. [28]. Here they integrated the structural genes from data of eight independent studies [64,65,66,67,68,69,70,71] and the regulatory genes from data of six independent studies [64,65,68,70,71,72]. The carrot genes included in this study are shown in the Supplementary Table S1
with their DCAR and/or LOC ID numbers based on the review of Iorizzo et al. [28].
2.1. Structural Genes
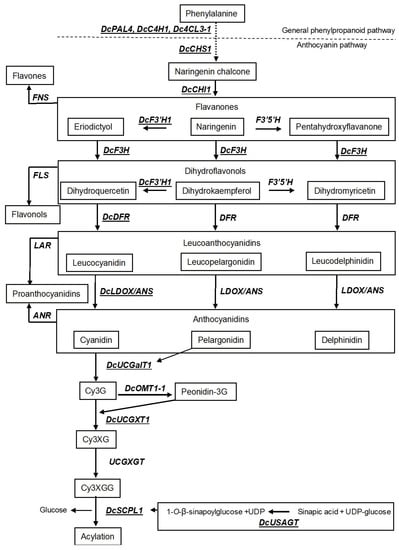
Anthocyanins are produced by a set of biosynthetic genes that are highly conserved across species in the plant kingdom [8][38]. The major flux is derived from general phenylpropanoid pathway and shikimate pathway, which is stepwise converted to anthocyanins, flavone, flavonols, proanthocyanins, and other phenolic compounds.
Anthocyanins are produced by a set of biosynthetic genes that are highly conserved across species in the plant kingdom [8,73]. The major flux is derived from general phenylpropanoid pathway and shikimate pathway, which is stepwise converted to anthocyanins, flavone, flavonols, proanthocyanins, and other phenolic compounds.
In brief, the general biosynthesis pathway leading to anthocyanidins in plants starts with the conversion of L-phenylalanine into trans-cinnamic acid by phenylalanine ammonia lyase (PAL
) as the first step of phenylpropanoid pathway. Cinnamate 4-hydroxylase (C4H
) and 4-coumaroyl-coenzyme A ligase (4CL
) further catalyze synthesis of p
-coumaric acid and p
-coumaroyl-Co-A, respectively. Three molecules of malonyl-Co-A derived from the shikimate pathway and one molecule of p
-coumaroyl-Co-A are then condensed to form naringenin chalcone by chalcone synthase (CHS
) (). Naringenin chalcone is then converted into naringenin catalyzed by chalcone isomerase (CHI
). Naringenin can be converted into the two other flavanones i.e., eriodictoyl and pentahydroxyflavanone by flavonoid 3′-hydroxylase (F3′H
) and flavonoid 3′-5′-hydroxylase (F3′5′H
), respectively, or catalyzed by flavanone 3- hydroxylase (F3H
) to dihydrokaempferol. Dihydrokaempferol can act as a substrate for both F3′H
and F3′5′H
to form dihydroquercetin and dihydromyricetin, respectively (). However, F3H
can also catalyze the formation of dihydroquercetin and dihydromyricetin from eriodictyol and pentahydroxyflavanone, respectively. The dihydroflavonols are then reduced by dihydroflavonol 4-reductase (DFR
) to corresponding leucoanthocyanidins. The colorless leucoanthocyanidins are oxidized to their corresponding colored anthocyanidins by leucoanthocyanidin dioxygenase (LDOX
), also known as anthocyanidin synthase (ANS
) (). The resultant anthocyanidins formed by LDOX
/ANS
are inherently unstable in cytosol and are immediately glycosylated (e.g., most commonly by UDP-glucose: flavonoid 3-O
-glucosyltransferase flavonoid glucosyltransferase: UFGT
), and these glycosylated products are sometimes further methylated (e.g., by O
-methyl transferase: OMT
) and sometimes also acylated (e.g., by anthocyanin acyltransferase: ACT) for stability as vacuolar anthocyanins [39].
) for stability as vacuolar anthocyanins [74].
Figure 2.
Simplified schematic diagram of the anthocyanin biosynthetic pathway. Structural enzymes are indicated in capital italic letters and intermediate compounds are represented in boxes. Enzymes underlined and with Dc prefix are upregulated in purple versus non-purple black carrot taproot tissue.
PAL
, phenylalanine ammonia lyase;
C4H
, cinnamate 4-hydroxylase;
4CL
, 4-coumaroyl-coenzyme A ligase;
CHS
, chalcone synthase;
CHI
, chalcone isomerase;
FNS
, flavone synthase;
F3H
, flavanone 3-hydroxylase;
FLS
, flavonol synthase;
F3′H
, flavonoid 3′-hydroxylase;
F3′5′H,
flavonoid 3′-5′-hydroxylase;
DFR
, dihydroflavonol 4-reductase;
LAR
, leucoanthocyanidin reductase;
ANR
, anthocyanidin reductase;
LDOX/ANS
, leucoanthocyanidin dioxygenase/anthocyanidin synthase;
UCGalT1
, UDP-galactose: cyanidin galactosyltransferase;
OMT
,
O
-methyl transferase;
UCGXT1
, UDP-xylose:cyanidin 3-galactoside xylosyltransferase;
UCGXGT1
, UDP-glucose:cyanidin 3-xylosylgalactoside glucosyltransferase;
SCPL
, serine carboxypeptidase-like;
USAGT1
, UDP-glucose: sinapic acid glucosyltransferase.
Bold arrows
indicate direct conversion.
Dashed arrow
indicates conversion through intermediates.
As previously mentioned, the predominant anthocyanins in the taproots of black carrots are derived from cyanidin. Recent studies in black carrots have identified structural genes involved in most of the steps leading to cyanidin synthesis [32][34]. These include the
As previously mentioned, the predominant anthocyanins in the taproots of black carrots are derived from cyanidin. Recent studies in black carrots have identified structural genes involved in most of the steps leading to cyanidin synthesis [67,69]. These include the DcPAL4, DcC4H1, Dc4CL3-1
genes of the general phenylpropanoid pathway providing the flux for the anthocyanin pathway and the DcCHS1, DcCHI1, DcF3H1, DcF3′H1, DcDFR1
, and DcLDOX1
/ANS
genes leading to cyanidin synthesis ().
Additionally, the UDP-glucose:cyanidin galactosyltransferase (DcUCGalT1) responsible for the initial glycosylation, which in the case of black carrot is a galactosylation of cyanidin to form cyanidin 3-galactoside (Cy3G) has been identified [40] as well as the enzyme responsible for the further glycosylation of Cy3G to cyanidin 3-xylosylgalactoside (Cy3XG) called UDP-xylose:cyanidin 3-galactoside xylosyltransferase (
) responsible for the initial glycosylation, which in the case of black carrot is a galactosylation of cyanidin to form cyanidin 3-galactoside (Cy3G) has been identified [75] as well as the enzyme responsible for the further glycosylation of Cy3G to cyanidin 3-xylosylgalactoside (Cy3XG) called UDP-xylose:cyanidin 3-galactoside xylosyltransferase (DcUCGXT1) [35]. However, the enzyme for the next glycosylation of Cy3XG to cyanidin 3-xylosyl (glucosyl) galactoside (Cy3XGG) called UDP-glucose:cyanidin 3-xylosylgalactoside glucosyltransferase (
) [70]. However, the enzyme for the next glycosylation of Cy3XG to cyanidin 3-xylosyl (glucosyl) galactoside (Cy3XGG) called UDP-glucose:cyanidin 3-xylosylgalactoside glucosyltransferase (UCGXGT) has not yet been identified. Cy3XGG is the substrate for acylation in black carrots [31][36] (
) has not yet been identified. Cy3XGG is the substrate for acylation in black carrots [66,71] (). Three types of mono-acylation cyanidin products are found in black carrots i.e., cyanidin 3-xylosyl (coumaroylglucosyl) galactoside (Cy3XCGG), cyanidin 3-xylosyl (feruloylglucosyl) galactoside (Cy3XFGG), and cyanidin 3-xylosyl (sinapoylglucosyl) galactoside (Cy3XSGG). The gene controlling the acylation of Cy3XGG to Cy3XSGG called serine carboxypeptidase-like 1
(DcSCPL1) was recently identified [31][36]. Correspondingly, the UDP-glucose:sinapic acid glucosyltransferase enzyme (
) was recently identified [66,71]. Correspondingly, the UDP-glucose:sinapic acid glucosyltransferase enzyme (DcUSAGT
) that transfers a glucose to the carboxyl group of sinapic acid forming 1-O-β-sinapoylglucose serving as an acyl donor to form Cy3XSGG was also recently identified [41]. The acylation results in the release of the glucose molecule from 1-
-β-sinapoylglucose serving as an acyl donor to form Cy3XSGG was also recently identified [76]. The acylation results in the release of the glucose molecule from 1-O
-β-sinapoylglucose ().
Moreover, one methyltransferase
gene called DcOMT1-1
has presently been identified in black carrots [28]. The expression of this gene has, however, not been found upregulated in any of the cultivars/mapping populations currently investigated probably due to the absence or low levels of peonidin in most black carrot cultivars.
As shown in , there are several enzymes along the anthocyanidin pathway competing with the direct anthocyanidin pathway. Firstly, flavanones can also act as a substrate for flavone synthase (FNS)
, which drives the anthocyanin pathway towards the biosynthesis of flavones with a yellowish color. Two DcFNS genes have been identified in black carrots and their expression levels were negatively correlated with anthocyanin concentrations in the carrot root phloem [29]. Secondly, the dihydroflavonols can also act as substrate of flavonol synthase (
genes have been identified in black carrots and their expression levels were negatively correlated with anthocyanin concentrations in the carrot root phloem [64]. Secondly, the dihydroflavonols can also act as substrate of flavonol synthase (FLS)
, which drives the anthocyanin pathway towards the biosynthesis of the colorless flavonols. Additionally, here, two DcFLS
genes have presently been identified in black carrots [28]. Thirdly, leucoanthocyanidins and anthocyanidins can also act as substrate for leucoanthocyanidin reductase (LAR
) and anthocyanidin reductase (ANR)
, respectively, driving the anthocyanin pathway towards the colorless proanthocyanidins. However, presently no LAR
or ANR
genes have been identified in black carrots [28].
2.2. Transcriptional Regulatory Activating Genes
The structural genes of anthocyanin biosynthesis in all plant species are under strict control of transcriptional regulatory genes [8][38]. The anthocyanin pathway is regulated by a ternary complex called the MBW-complex that consists of three transcription factors (TFs) i.e., a R2R3-MYB TF, a basic helix-loop-helix (bHLH) TF and a WD40 repeat protein [42]. In general, the transcription levels of R2R3-MYB TFs and bHLH TFs differ among organs, tissues and cell types and in response to environmental conditions [43] while WD40 seems to be transcribed constitutively in all cell types [44]. The R2R3-MYB TFs are the major contributors to the anthocyanin pathway regulation [45]. These contain the two highly conserved DNA binding domain repeats (R2 and R3) in the N-terminal and a more variable non-MYB region in the C-terminal containing the transcriptional regulation domain [46]. Besides, the R3-MYB repeat contains a bHLH-binding domain that binds the bHLH coactivator and both of these activators interact with a WD40 protein to form the MBW-complex. The R2R3-MYB and the bHLH proteins of the MBW-complex interact directly with the promoter of target genes activating the transcription of structural genes in the anthocyanin pathway [14][42][46].
The structural genes of anthocyanin biosynthesis in all plant species are under strict control of transcriptional regulatory genes [8,73]. The anthocyanin pathway is regulated by a ternary complex called the MBW-complex that consists of three transcription factors (TFs) i.e., a R2R3-MYB TF, a basic helix-loop-helix (bHLH) TF and a WD40 repeat protein [77]. In general, the transcription levels of R2R3-MYB TFs and bHLH TFs differ among organs, tissues and cell types and in response to environmental conditions [78] while WD40 seems to be transcribed constitutively in all cell types [79]. The R2R3-MYB TFs are the major contributors to the anthocyanin pathway regulation [80]. These contain the two highly conserved DNA binding domain repeats (R2 and R3) in the N-terminal and a more variable non-MYB region in the C-terminal containing the transcriptional regulation domain [81]. Besides, the R3-MYB repeat contains a bHLH-binding domain that binds the bHLH coactivator and both of these activators interact with a WD40 protein to form the MBW-complex. The R2R3-MYB and the bHLH proteins of the MBW-complex interact directly with the promoter of target genes activating the transcription of structural genes in the anthocyanin pathway [14,77,81].
In black carrot, candidate genes for anthocyanin related R2R3-MYB TFs were only recently identified. It has been known for quite some time that QTLs for the genetic control of anthocyanin biosynthesis in black carrots could be assigned to two regions on chromosome 3 called P1 and P3 located relatively close but more than 30 cM apart [30][32][47]. However, recently R2R3-MYB TF genes were identified within these regions [29][30]. A cluster of 6 anthocyanin related R2R3-MYB genes located within the P3 region and one R2R3-MYB gene located within the P1 region were identified [29][30]. The R2R3-MYB TF genes located within the P3 region were called
In black carrot, candidate genes for anthocyanin related R2R3-MYB TFs were only recently identified. It has been known for quite some time that QTLs for the genetic control of anthocyanin biosynthesis in black carrots could be assigned to two regions on chromosome 3 called P1 and P3 located relatively close but more than 30 cM apart [65,67,82]. However, recently R2R3-MYB TF genes were identified within these regions [64,65]. A cluster of 6 anthocyanin related R2R3-MYB genes located within the P3 region and one R2R3-MYB gene located within the P1 region were identified [64,65]. The R2R3-MYB TF genes located within the P3 region were called DcMYB6
, DcMYB7
, DcMYB8
, DcMYB9
, DcMYB10
, and DcMYB11
and the R2R3-MYB TF located within the P1 region was called DcMYB12
.
The expression level of DcMYB7 was found to be highly correlated with taproot anthocyanin pigmentation in the mapping populations used to identify the cluster of R2R3-MYB TFs in the P3 region [30]. Moreover, the expression of
was found to be highly correlated with taproot anthocyanin pigmentation in the mapping populations used to identify the cluster of R2R3-MYB TFs in the P3 region [65]. Moreover, the expression of DcMYB7 gene has also been found highly upregulated in purple taproot tissue versus non-purple taproot tissue in several other studies of different black carrot cultivars [29][33][35][37]. Further proof that
gene has also been found highly upregulated in purple taproot tissue versus non-purple taproot tissue in several other studies of different black carrot cultivars [64,68,70,72]. Further proof that DcMYB7
is the R2R3-MYB TF determining anthocyanin pigmentation in some black carrot cultivars was obtained by knocking out the DcMYB7 in the purple carrot cultivar Deep Purple by CRISPR/Cas9 [35][48]. This resulted in taproots that were yellow in the entire taproot.
in the purple carrot cultivar Deep Purple by CRISPR/Cas9 [70,83]. This resulted in taproots that were yellow in the entire taproot.
However, other studies have shown that DcMYB7 is not upregulated in the purple taproot tissue in some black carrot cultivars [33][37]. In one study,
However, other studies have shown that DcMYB7 is not upregulated in the purple taproot tissue in some black carrot cultivars [68,72]. In one study, DcMYB7 was only found upregulated in purple versus non-purple taproot tissue in the cultivar CH5544 but not in the cultivar Night Bird also included in that study [37]. Likewise, another study showed that
was only found upregulated in purple versus non-purple taproot tissue in the cultivar CH5544 but not in the cultivar Night Bird also included in that study [72]. Likewise, another study showed that DcMYB7 was not expressed in the purple taproot tissue of the cultivar Purple Haze [33]. This strongly indicates that
was not expressed in the purple taproot tissue of the cultivar Purple Haze [68]. This strongly indicates that DcMYB7 is not controlling anthocyanin biosynthesis in these cultivars and could indicate that the R2R3-MYB factors responsible for anthocyanin biosynthesis in black carrots differ between cultivars. Furthermore, a different R2R3-MYB TF was recently identified in Purple Haze [36]. This R2R3-MYB TF was only expressed in the purple taproot tissue of Purple Haze but not expressed in the two black carrot control cultivars of that study i.e., Deep Purple and Cosmic Purple where
is not controlling anthocyanin biosynthesis in these cultivars and could indicate that the R2R3-MYB factors responsible for anthocyanin biosynthesis in black carrots differ between cultivars. Furthermore, a different R2R3-MYB TF was recently identified in Purple Haze [71]. This R2R3-MYB TF was only expressed in the purple taproot tissue of Purple Haze but not expressed in the two black carrot control cultivars of that study i.e., Deep Purple and Cosmic Purple where DcMYB7 is upregulated in the purple taproot tissue [36]. This R2R3-MYB TF gene was named
is upregulated in the purple taproot tissue [71]. This R2R3-MYB TF gene was named DcMYB113
and it is corresponding to the DcMYB12 located in the P1 region [29].
located in the P1 region [64].
Although the R2R3-MYB TFs responsible for anthocyanin biosynthesis have now been identified in several black carrot cultivars, it is also important to identify the corresponding bHLH partners that can bind to the R3-MYB of these R2R3-MYB TFs. The DcbHLH3
gene located on chromosome 1 has been suggested as bHLH partner in several studies. DcbHLH3
was highly upregulated in purple versus non-purple tissue together with DcMYB7 in the cultivar CH5544 [37]. Similarly,
in the cultivar CH5544 [72]. Similarly, DcbHLH3
was also found to be highly upregulated in the orange carrot Kurodagosun transformed with either DcMYB7
or DcMYB113 [35][36] indicating that the bHLH partner is the same for both R2R3-MYB TFs and that both
[70,71] indicating that the bHLH partner is the same for both R2R3-MYB TFs and that both DcMYB7
and DcMYB113
can upregulate the expression of DcbHLH3
.
Only one WD40 transcript has been detected in carrot roots [28]. This WD40 is named DcTTG1
since it has homology to Arabidopsis AtTTG1 that is a constant member of the MBW complex required for the activation of the anthocyanin pathway in Arabidopsis [37]. Like in Arabidopsis, the
that is a constant member of the MBW complex required for the activation of the anthocyanin pathway in Arabidopsis [72]. Like in Arabidopsis, the DcTTG1 gene was found to be constitutively expressed in black carrots [30][31]. Thus,
gene was found to be constitutively expressed in black carrots [65,66]. Thus, DcTTG1
was proposed as a possible candidate for the formation of the MBW complex regulating anthocyanin biosynthesis in black carrot taproots [28].
3. Induction of the Anthocyanin Pathway in Orange Carrot
Early carrots were purple and yellow and arose from Central Asia [49]. Both purple and yellow carrots were imported to Europe and the yellow carrot became increasingly popular in Europe. The yellow carrot is thought to have formed the genetic basis for the selection of the first white and orange carrots [50]. Orange carrots (being orange due to the high content of carotenoids) are currently much more adapted to Western climate than black carrots because of breeding for this climate through centuries. One very important difference between the current purple and orange cultivars is that purple carrot has a higher tendency to flower already in the first season causing no or little taproot development and thereby a very low yield [51].
Early carrots were purple and yellow and arose from Central Asia [107]. Both purple and yellow carrots were imported to Europe and the yellow carrot became increasingly popular in Europe. The yellow carrot is thought to have formed the genetic basis for the selection of the first white and orange carrots [108]. Orange carrots (being orange due to the high content of carotenoids) are currently much more adapted to Western climate than black carrots because of breeding for this climate through centuries. One very important difference between the current purple and orange cultivars is that purple carrot has a higher tendency to flower already in the first season causing no or little taproot development and thereby a very low yield [109].
Recent research has shown that the non-purple carrots appear to have unfunctional anthocyanin activator TF regulatory genes. This has been revealed by inserting the DcMYB7 gene controlled by the constitutive 35S-promoter into the orange carrot cultivar Kurodagosun that turned the orange Kurodagosun taproot into a purple taproot producing anthocyanins in all tissues of the taproot [35]. Likewise, the simultaneous insertion of the snapdragon R2R3-MYB
gene controlled by the constitutive 35S-promoter into the orange carrot cultivar Kurodagosun that turned the orange Kurodagosun taproot into a purple taproot producing anthocyanins in all tissues of the taproot [70]. Likewise, the simultaneous insertion of the snapdragon R2R3-MYB AmRosea
gene and the bHLH AmDelila gene controlled by the 35S-promoter into the orange carrot Danvers turned the taproots into purple taproots producing anthocyanins in all tissues of the taproot [52]. Very recently, the
gene controlled by the 35S-promoter into the orange carrot Danvers turned the taproots into purple taproots producing anthocyanins in all tissues of the taproot [34]. Very recently, the DcMYB113 gene (identified in Purple Haze) was inserted into Kurodagosun controlled by the 35S-promoter. This also turned the orange taproots into purple taproots producing anthocyanins in all tissues of the taproot [36].
gene (identified in Purple Haze) was inserted into Kurodagosun controlled by the 35S-promoter. This also turned the orange taproots into purple taproots producing anthocyanins in all tissues of the taproot [71].
These studies all showed that the anthocyanin profile of the purple converted orange carrots was similar to black carrot cultivars confirming that the structural genes of the anthocyanin biosynthesis pathway was still intact in the orange carrots. Thus, it might be possible to not only turn the more well-adapted orange carrots into purple carrots but also to use the same approaches as described for black cultivars to generate carrots with different colors, increased acylation or increased total anthocyanin content (). However, the transformation constructs used for changing the anthocyanin composition in black carrots have to be combined with genes for the regulatory TFs inducing anthocyanin biosynthesis in orange carrots.