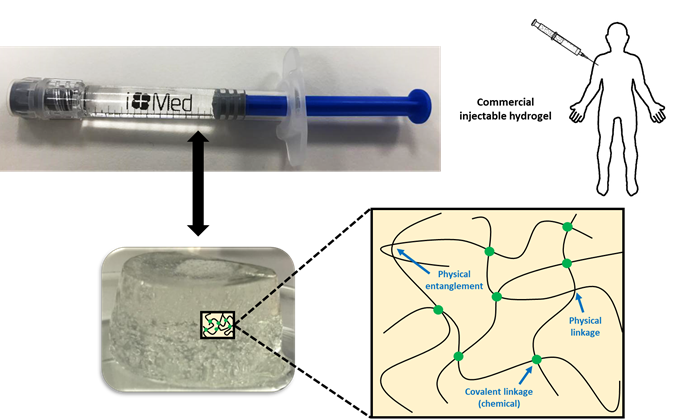
The transfer of some innovative technologies from the laboratory to industrial scale is many times not taken into account in the design and development of some functional materials such as hydrogels to be applied in the biomedical field. There is a lack of knowledge in the scientific field where many aspects of scaling to an industrial process are ignored, and products cannot reach the market. Injectable hydrogels are a good example that we have used in our research to show the different steps needed to follow to get a product in the market based on them. From synthesis and process validation to characterization techniques used and assays performed to ensure the safety and efficacy of the product, following regulation, several well-defined proto-cols must be adopted.
- injectable
- hydrogels
- industrialization
- scale-up
- polysaccharides
- regulation
Note: The following contents are extract from your paper. The entry will be online only after author check and submit it.
1. Introduction
Hydrogels are three-dimensional physically or chemically crosslinked polymeric networks from natural or synthetic origin, with an intrinsic hydrophilic character due to their functional groups. They have unique properties, such as high water content, softness, flexibility, porosity, permeability, and biocompatibility and a very high affinity for water and other body fluids. These properties resemble those of many soft living tissues, which opens up many opportunities in biomedical and pharmaceutical applications [1,2]. In this regard, drug delivery systems based on hydrogels have been developed in order to provide drug reservoirs for treating conditions of soft tissues such as for example bladder diseases [3]. Similarly hydrogels have been synthetized as scaffolds for cartilage tissue engineering [4]. Therefore, these properties make hydrogels an ideal candidate to provide a better adaptation after the implantation.
Hydrogels are three-dimensional physically or chemically crosslinked polymeric networks from natural or synthetic origin, with an intrinsic hydrophilic character due to their functional groups. They have unique properties, such as high water content, softness, flexibility, porosity, permeability, and biocompatibility and a very high affinity for water and other body fluids. These properties resemble those of many soft living tissues, which opens up many opportunities in biomedical and pharmaceutical applications [1][2]. In this regard, drug delivery systems based on hydrogels have been developed in order to provide drug reservoirs for treating conditions of soft tissues such as for example bladder diseases [3]. Similarly hydrogels have been synthetized as scaffolds for cartilage tissue engineering [4]. Therefore, these properties make hydrogels an ideal candidate to provide a better adaptation after the implantation.
In some cases, chemical and physical networks might coexist in one hydrogel (Figure 1). Physical hydrogels are formed by reversible physics interactions, and they can be dissolved by changing environmental conditions [5]. Otherwise, chemical hydrogels are mainly formed by the covalent bonds that appear after certain chemical reactions, and they are recognized as stable or permanent in physiological conditions. These hydrogels can be prepared by using a hydrophilic monomer polymerized in the presence of a polyfunctional crosslinking agent [6] or by the direct crosslinking of water-soluble monomers in the presence of a free-radical generating initiator that can be activated by radiation (light, heat, etc.) or by chemical reactions (redox). However, some of these pathways often result in materials containing significant levels of residual unreacted monomers that are often toxic and could lixiviate out from the hydrogel. Thus, a purification step is needed, and this can take up from several days to weeks to be completed. The selection of non-toxic oligomers or macromonomers (e.g., polyethylene glycol acrylate derivatives) could be an alternative [7].
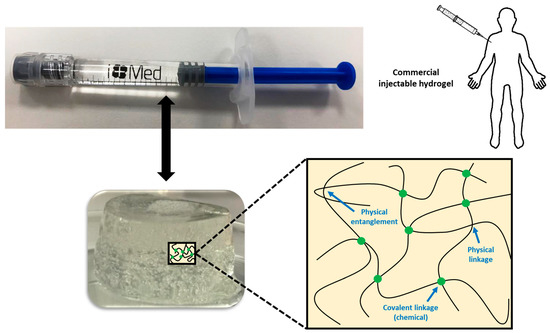
Figure 1. Physical interactions and chemical linkages of the chemical structure of an injectable hydrogel.
It is also possible to avoid the need for purification by using water-soluble polymers such as many polysaccharides. However, sometimes, they also need some kind of crosslinking agent, and the need to purify unreacted molecules of it comes back again. Among them, the most common glycosaminoglycan found in many living tissues is hyaluronic acid (HA) or hyaluronan. HA is a linear glycosaminoglycan made up of repeating units of N-acetyl-d-glucosamine and d-glucuronic acid with the monosaccharide units linked together via alternating -1,3 and -1,4 glycosidic bonds [8]. In physiological conditions, HA takes the form of a sodium salt that is negatively charged and highly hydrophilic [9]. HA can be crosslinked by several of the methods mentioned before to form hydrogels for several biomedical applications. However, the implantation of pre-formed hydrogels at a desired site in the body demands an invasive surgical procedure that can cause the patient’s pain and discomfort as well as the cost and time [10][11]. For this reason, injectable hydrogels are becoming more interesting for many of these applications.
It is also possible to avoid the need for purification by using water-soluble polymers such as many polysaccharides. However, sometimes, they also need some kind of crosslinking agent, and the need to purify unreacted molecules of it comes back again. Among them, the most common glycosaminoglycan found in many living tissues is hyaluronic acid (HA) or hyaluronan. HA is a linear glycosaminoglycan made up of repeating units of N-acetyl-d-glucosamine and d-glucuronic acid with the monosaccharide units linked together via alternating –1,3 and –1,4 glycosidic bonds [8]. In physiological conditions, HA takes the form of a sodium salt that is negatively charged and highly hydrophilic [9]. HA can be crosslinked by several of the methods mentioned before to form hydrogels for several biomedical applications. However, the implantation of pre-formed hydrogels at a desired site in the body demands an invasive surgical procedure that can cause the patient’s pain and discomfort as well as the cost and time [10,11]. For this reason, injectable hydrogels are becoming more interesting for many of these applications.
As hydrogels are becoming more useful materials in many therapeutical approaches, the challenge has arisen for research laboratories and the biomedical industry to get an effective technology transfer and scale-up of processes. For this reason, many aspects that affect the scaling to an industrial process should be considered right from the beginning of any new development. This industry-focused point of view is ignored by scientists most of the time, and that is one of the reasons why many good ideas and potential products never reach the market, and thus, the patient, who would never benefit from them. While the exploration on synthesis is indispensable, the construction of new formulations using common materials, simple methods, and facile design must become a preferential choice, without forgetting to assess the influence of the process procedure on the properties of the fabricated hydrogel. Not just the chemical, physical, and biological parameters must be controlled in order to guarantee a successful process, but also economic, legal, and regulatory aspects. The product obtained in the laboratory should have not only the same characteristics and performance as that at the larger scale but also must be obtained by reproducible and controllable processes. From the point of view of regulation, the biomedical field is very strict in parameters such as sterility and toxicity.
As hydrogels are becoming more useful materials in many therapeutical approaches, the challenge has arisen for research laboratories and the biomedical industry to get an effective technology transfer and scale-up of processes. For this reason, many aspects that affect the scaling to an industrial process should be considered right from the beginning of any new development. This industry-focused point of view is ignored by scientists most of the time, and that is one of the reasons why many good ideas and potential products never reach the market, and thus, the patient, who would never benefit from them. While the exploration on synthesis is indispensable, the construction of new formulations using common materials, simple methods, and facile design must become a preferential choice, without forgetting to assess the influence of the process procedure on the properties of the fabricated hydrogel. Not just the chemical, physical, and biological parameters must be controlled in order to guarantee a successful process, but also economic, legal, and regulatory aspects. The product obtained in the laboratory should have not only the same characteristics and performance as that at the larger scale but also must be obtained by reproducible and controllable processes. From the point of view of regulation, the biomedical field is very strict in parameters such as sterility and toxicity.
For example, in the characterization of injectable hydrogels, several parameters must be determined, since they are necessary for their industrialization. These requirements are listed in European Pharmacopoeia (Ph. Eur.) and should follow International Organization for Standardization (ISO) norms or American Society for Testing Materials (ASTM) standards. Therefore, they must possess a list of specifications (characteristics of the commercial product) with a description of validated analytical techniques used for the measurement of these specifications. Thus, to get the CE marking and the consequent commercialization of the product, the list of specifications of the injectable product must conform the established and tolerable limits for its future application.
For example, in the characterization of injectable hydrogels, several parameters must be determined, since they are necessary for their industrialization. These requirements are listed in European Pharmacopoeia (Ph. Eur.) and should follow International Organization for Standardization (ISO) norms or American Society for Testing Materials (ASTM) standards. Therefore, they must possess a list of specifications (characteristics of the commercial product) with a description of validated analytical techniques used for the measurement of these specifications. Thus, to get the CE marking and the consequent commercialization of the product, the list of specifications of the injectable product must conform the established and tolerable limits for its future application.
This paper summarizes all these aspects and shows a real example of a development of a natural crosslinked hydrogel based on hyaluronic acid that reached the market in the form of an injectable product.
This paper summarizes all these aspects and shows a real example of a development of a natural crosslinked hydrogel based on hyaluronic acid that reached the market in the form of an injectable product.
2. Injectable Hydrogels: Properties and Synthesis Techniques
2. Injectable Hydrogels: Properties and Synthesis Techniques
Injectable hydrogels have the right physicochemical properties to be injected in situ into the body and therefore, they have attracted significant interest in drug delivery, tissue engineering, and as dermal fillers [12][13][14][15]. Thus, one of the most important factors to be considered is the viscosity of the polymer solution, as this feature is advantageous in minimally invasive surgical procedures. Some injected hydrogels trigger a wide variety of inflammatory, immune-mediated, local, or systemic adverse reactions that appear early or late, illustrating that biocompatibility and non-toxicity are important criteria of a good injectable hydrogel system. Another important factor is the hydrogel porosity, where highly inter-connected networks are preferred, as they facilitate better movement of nutrients and adaptation to surrounding tissues. This also relates to the proper mechanical properties (tensile strength and modulus, compressive stress and modulus, shear stress, stiffness, storage and loss moduli, fragility, mesh size, and density, among others) [16], as the hydrogel should withstand the repetitive deformation that occurs in the mechanically dynamic environment in the body.
Injectable hydrogels have the right physicochemical properties to be injected in situ into the body and therefore, they have attracted significant interest in drug delivery, tissue engineering, and as dermal fillers [12–15]. Thus, one of the most important factors to be considered is the viscosity of the polymer solution, as this feature is advantageous in minimally invasive surgical procedures. Some injected hydrogels trigger a wide variety of inflammatory, immune-mediated, local, or systemic adverse reactions that appear early or late, illustrating that biocompatibility and non-toxicity are important criteria of a good injectable hydrogel system. Another important factor is the hydrogel porosity, where highly inter-connected networks are preferred, as they facilitate better movement of nutrients and adaptation to surrounding tissues. This also relates to the proper mechanical properties (tensile strength and modulus, compressive stress and modulus, shear stress, stiffness, storage and loss moduli, fragility, mesh size, and density, among others) [16], as the hydrogel should withstand the repetitive deformation that occurs in the mechanically dynamic environment in the body.
An injectable hydrogel is generally based on the idea that some biomaterials can be injected as liquid into human body, and then, an in situ solid hydrogel is formed [17]. However, injectable hydrogels are not only all those that gel once they have been injected into the human body. Indeed, hydrogels with shear-tinning and self-healing properties are also recognized as injectable biomaterials, since they can also be injected directly into the gel state [18][19][20]. All in all, these injectable biomaterials require a better control of gelation kinetics on the process procedure, which requires transporting the sol or the pre-gel to a targeting site through an injection device. They should fill the void space and especially have the desired stability at the injected site. This means that the sol–gel transition of an injectable hydrogel should happen within a restricted time interval to get the right injectability. Moreover, the injection procedure for injectable hydrogels could also influence the structure and the properties of the harvested bulk gel, reaching a poorer performance against deformation than the corresponding in situ formed gels. However, as in other hydrogels, the mechanical properties and durability can be tuned by varying the portion of the monomers or oligomers, the molecular weights, and the crosslinking density of the hydrogel.
An injectable hydrogel is generally based on the idea that some biomaterials can be injected as liquid into human body, and then, an in situ solid hydrogel is formed [17]. However, injectable hydrogels are not only all those that gel once they have been injected into the human body. Indeed, hydrogels with shear-tinning and self-healing properties are also recognized as injectable biomaterials, since they can also be injected directly into the gel state [18–20]. All in all, these injectable biomaterials require a better control of gelation kinetics on the process procedure, which requires transporting the sol or the pre-gel to a targeting site through an injection device. They should fill the void space and especially have the desired stability at the injected site. This means that the sol–gel transition of an injectable hydrogel should happen within a restricted time interval to get the right injectability. Moreover, the injection procedure for injectable hydrogels could also influence the structure and the properties of the harvested bulk gel, reaching a poorer performance against deformation than the corresponding in situ formed gels. However, as in other hydrogels, the mechanical properties and durability can be tuned by varying the portion of the monomers or oligomers, the molecular weights, and the crosslinking density of the hydrogel.
The typical crosslinking strategies applicable for the synthesis of ordinary hydrogels are also applied for the development of injectable hydrogels. So far, injectable hydrogels can be crosslinked by different synthesis mechanisms, and in their chemical structure, physical and chemical linkages can be found and coexist between them [21][22]. Physical linkages include electrostatic interactions such as electrostatic forces, ionic and hydrogen bonds, van der Waals forces, π-interactions, or hydrophobic interactions. Hydrogels that only or mainly possess physical interactions in their structure can be merely prepared without any additional reactive reagents, but they are slightly mechanically weak to preserve themselves from the ambient influences such as pHs and temperatures for a long time, when injected into the body. On the other hand, in injectable hydrogels in which chemical linkages are majority and dominant forces Diels–Alder reactions, Michael-type additions, Schiff base reactions, enzyme-mediations, thiol exchange/disulfide crosslinking, and click chemistry can be included. These hydrogels can be triggered by external stimuli (temperature, pH, light, electric/magnetic fields, ultrasound, and enzymes), and they have been shown to possess relatively higher mechanical stability and physicochemical properties with greater durability over time due to the formed strong covalent bonds.
The typical crosslinking strategies applicable for the synthesis of ordinary hydrogels are also applied for the development of injectable hydrogels. So far, injectable hydrogels can be crosslinked by different synthesis mechanisms, and in their chemical structure, physical and chemical linkages can be found and coexist between them [21,22]. Physical linkages include electrostatic interactions such as electrostatic forces, ionic and hydrogen bonds, van der Waals forces, π-interactions, or hydrophobic interactions. Hydrogels that only or mainly possess physical interactions in their structure can be merely prepared without any additional reactive reagents, but they are slightly mechanically weak to preserve themselves from the ambient influences such as pHs and temperatures for a long time, when injected into the body. On the other hand, in injectable hydrogels in which chemical linkages are majority and dominant forces Diels–Alder reactions, Michael-type additions, Schiff base reactions, enzyme-mediations, thiol exchange/disulfide crosslinking, and click chemistry can be included. These hydrogels can be triggered by external stimuli (temperature, pH, light, electric/magnetic fields, ultrasound, and enzymes), and they have been shown to possess relatively higher mechanical stability and physicochemical properties with greater durability over time due to the formed strong covalent bonds.
In general, HA-based injectable hydrogels are prepared using chemical crosslinking agents such as 1,4-butanediol diglycidyl ether (BDDE) and divinyl sulfone (DVS) to overcome the very short half-life of HA, several hours in the body, due to its fast enzymatic degradation by hyaluronidase [23]. However, despite the use of chemical crosslinking agents, the stability and longevity of these hydrogels usually shows a persistence of only 6 months in vivo, and they require repeated injection to maintain their efficacy [24][25]. Therefore, there is an unmet need to develop new injectable materials that are safer and longer lasting.
In general, HA-based injectable hydrogels are prepared using chemical crosslinking agents such as 1,4-butanediol diglycidyl ether (BDDE) and divinyl sulfone (DVS) to overcome the very short half-life of HA, several hours in the body, due to its fast enzymatic degradation by hyaluronidase [23]. However, despite the use of chemical crosslinking agents, the stability and longevity of these hydrogels usually shows a persistence of only 6 months in vivo, and they require repeated injection to maintain their efficacy [24,25]. Therefore, there is an unmet need to develop new injectable materials that are safer and longer lasting.
References
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23.
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267.
- Qiu, H.; Guo, H.; Li, D.; Hou, Y.; Kuang, T.; Ding, J. Intravesical Hydrogels as Drug Reservoirs. Trends Biotechnol. 2020, 38, 579–583.
- Zhang, Y.; Yu, J.; Ren, K.; Zuo, J.; Ding, J.; Chen, X. Thermosensitive Hydrogels as Scaffolds for Cartilage Tissue Engineering. Biomacromolecules 2019, 20, 1478–1492.
- Maiz-Fernández, S.; Pérez-álvarez, L.; Ruiz-Rubio, L.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. Polysaccharide-based in situ self-healing hydrogels for tissue engineering applications. Polymers (Basel) 2020, 12, 2261.
- Balakrishnan, B.; Banerjee, R. Biopolymer-Based Hydrogels for Cartilage Tissue Engineering. Chem. Rev. 2011, 111, 4453–4474.
- Lin-Gibson, S.; Bencherif, S.; Cooper, J.A.; Wetzel, S.J.; Antonucci, J.M.; Vogel, B.M.; Horkay, F.; Washburn, N.R. Synthesis and characterization of PEG dimethacrylates and their hydrogels. Biomacromolecules 2004, 5, 1280–1287.
- Vasi, A.M.; Popa, M.I.; Butnaru, M.; Dodi, G.; Verestiuc, L. Chemical functionalization of hyaluronic acid for drug delivery applications. Mat. Sci. Eng. 2014, 38, 177–185.
- Rice, K.G. The Chemistry, Biology, and Medical Applications of Hyaluronan and Its Derivatives; Laurent, T.C., Ed.; Portland Press: London, UK, 1998.
- Piantanida, E.; Alonci, G.; Bertucci, A.; De Cola, L. Design of Nanocomposite Injectable Hydrogels for Minimally Invasive Surgery. Acc. Chem. Res. 2019, 52, 2101–2112.
- Salzlechner, C.; Haghighi, T.; Huebscher, I.; Walther, A.R.; Schell, S.; Gardner, A.; Undt, G.; da Silva, R.M.P.; Dreiss, C.A.; Fan, K.; et al. Adhesive Hydrogels for Maxillofacial Tissue Regeneration Using Minimally Invasive Procedures. Adv. Healthc. Mater. 2020, 9.
- Hyun, H.; Park, M.H.; Lim, W.; Kim, S.Y.; Jo, D.; Jung, J.S.; Jo, G.; Um, S.; Lee, D.W.; Yang, D.H. Injectable visible light-cured glycol chitosan hydrogels with controlled release of anticancer drugs for local cancer therapy in vivo: A feasible study. Artif. Cells Nanomed. Biotechnol. 2018, 46, 874–882.
- 13. Pertici, V.; Pin-Barre, C.; Rivera, C.; Pellegrino, C.; Laurin, J.; Gigmes, D.; Trimaille, T. Degradable and Injectable Hydrogel for Drug Delivery in Soft Tissues. Biomacromolecules 2019, 20, 149–163.
- Ren, B.; Chen, X.; Du, S.; Ma, Y.; Chen, H.; Yuan, G.; Li, J.; Xiong, D.; Tan, H.; Ling, Z.; et al. Injectable polysaccharide hydrogel embedded with hydroxyapatite and calcium carbonate for drug delivery and bone tissue engineering. Inter. J. Bio. Macrom. 2018, 118, 1257–1266.
- Choi, W.I.; Hwang, Y.; Sahu, A.; Min, K.; Sung, D.; Tae, G.; Chang, J.H. An injectable and physical levan-based hydrogel as a dermal filler for soft tissue augmentation. Biomater. Sci. 2018, 6, 2627–2638.
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013, 92, 1262–1279.
- Deyhle, H.; Schulz, G.; Bert, M. Encyclopedia of Nanotechnology—Injectable Hydrogels; Springer Netherlands: Dordrecht, The Netherlands, 2012; ISBN 9789048197514.
- Uman, S.; Dhand, A.; Burdick, J.A. Recent advances in shear-thinning and self-healing hydrogels for biomedical applications. J. Appl. Polym. Sci. 2020, 137, 1–20.
- Mellati, A.; Akhtari, J. Injectable Hydrogels: A Review of Injectability Mechanisms and Biomedical Applications. Res. Mol. Med. 2019, 6, 1–19.
- Loebel, C.; Rodell, C.B.; Chen, M.H.; Burdick, J.A. Shear-thinning and self-healing hydrogels as injectable therapeutics and for 3D-printing. Nat. Protoc. 2017, 12, 1521–1541.
- Le, T.M.D.; Jung, B.K.; Li, Y.; Duong, H.T.T.; Nguyen, T.L.; Hong, J.W.; Yun, C.O.; Lee, D.S. Physically crosslinked injectable hydrogels for long-term delivery of oncolytic adenoviruses for cancer treatment. Biomater. Sci. 2019, 7, 4195–4207.
- Lee, J.H. Injectable hydrogels delivering therapeutic agents for disease treatment and tissue engineering. Biomater. Res. 2018, 22, 1–14.
- La Gatta, A.; Schiraldi, C.; Papa, A.; De Rosa, M. Comparative analysis of commercial dermal fillers based on crosslinked hyaluronan: Physical characterization and in vitro enzymatic degradation. Polym. Degrad. Stab. 2011, 96, 630–636.
- Buck, D.W.; Alam, M.; Kim, J.Y.S. Injectable fillers for facial rejuvenation: A review. J. Plast. Reconstr. Aesthetic Surg. 2009, 62, 11–18.
- Kim, Z.H.; Lee, Y.; Kim, S.M.; Kim, H.; Yun, C.K.; Choi, Y.S. A composite dermal filler comprising cross-linked hyaluronic acid and human collagen for tissue reconstructions. J. Microbiol. Biotechnol. 2014, 25, 399–406.