The nearly ubiquitous expression of endogenous 24 h oscillations known as circadian rhythms regulate the timing of physiological functions in the body. These intrinsic rhythms are sensitive to external cues, known as
zeitgebers
, which entrain the internal biological processes to the daily environmental changes in light, temperature, and food availability. Light directly entrains the master clock, the suprachiasmatic nucleus (SCN) which lies in the hypothalamus of the brain and is responsible for synchronizing internal rhythms. However, recent evidence underscores the importance of other hypothalamic nuclei in regulating several essential rhythmic biological functions. These extra-SCN hypothalamic nuclei also express circadian rhythms, suggesting distinct regions that oscillate either semi-autonomously or independent of SCN innervation. Concurrently, the extra-SCN hypothalamic nuclei are also sensitized to fluctuations in nutrient and hormonal signals. Thus, food intake acts as another powerful entrainer for the hypothalamic oscillators’ mediation of energy homeostasis. Ablation studies and genetic mouse models with perturbed extra-SCN hypothalamic nuclei function reveal their critical downstream involvement in an array of functions including metabolism, thermogenesis, food consumption, thirst, mood and sleep. Large epidemiological studies of individuals whose internal circadian cycle is chronically disrupted reveal that disruption of our internal clock is associated with an increased risk of obesity and several neurological diseases and disorders.
- circadian rhythm
- clock genes
- hypothalamus
- extra-SCN hypothalamic nuclei
- metabolism
- food-entrainable oscillator
- obesity
1. Introduction
Most organisms on earth exhibit highly conserved 24 h rhythms in physiology and behavior. Constant 24 h rotations of the earth punctuated by the rising and setting of the sun contribute to an organism’s circadian (i.e., 24 h) biology at the molecular, cellular, and behavioral levels. This internal clock not only sensitizes, but enables an organism to anticipate daily fluctuations in its environment. This time-keeping process operates in almost all cells of an organism and is self-perpetuating, even in the absence of external cues [1]. Although circadian clocks throughout the body are synchronized in large part through the suprachiasmatic nucleus (SCN) of the hypothalamus, rhythmicity in other hypothalamic nuclei has proved to be a critical regulator of physiological rhythms such as the sleep–wake cycle and daily food intake.
The circadian clock in the hypothalamus and elsewhere ultimately depends on 24 h rhythms at the cellular level, where a central transcription–translation feedback loop (TTFL) regulates the expression of key clock transcription factors (TFs). The core loop is a heterodimer consisting of circadian locomotor output cycles kaput (CLOCK) and brain and muscle ARNT-like protein (BMAL1), which promote the rhythmic expression of numerous E-Box-containing output genes, including the Period (Per 1–3) and Cryptochrome (Cry 1–2) genes, which as proteins function as direct repressors of the CLOCK:BMAL1 heterodimer [1,2]. Resumption of CLOCK:BMAL1 activity occurs only when these repressors are degraded by regulators such as the serine/threonine casein kinases (CK1δ and CK1ϵ), which phosphorylate PER, initiating its ubiquitination. CRY turnover is also controlled by phosphorylation; the metabolic sensor 5′ adenosine monophosphate-activated protein kinase (AMPK) tags it for proteasome degradation by direct phosphorylation. Additional loops consisting of the nuclear receptor subfamily 1 D member 1 (NR1D1) also known as REV-ERα/β and the retinoic acid receptor-related orphan receptors (RORs) sustain this core transcriptional loop by transactivating or repressing Bmal1 [3,4,5]. An integral non-circadian loop intertwined with the core clock includes the circadian metabolite nicotinamide adenine dinucleotide (NAD
The circadian clock in the hypothalamus and elsewhere ultimately depends on 24 h rhythms at the cellular level, where a central transcription–translation feedback loop (TTFL) regulates the expression of key clock transcription factors (TFs). The core loop is a heterodimer consisting of circadian locomotor output cycles kaput (CLOCK) and brain and muscle ARNT-like protein (BMAL1), which promote the rhythmic expression of numerous E-Box-containing output genes, including the Period (Per 1–3) and Cryptochrome (Cry 1–2) genes, which as proteins function as direct repressors of the CLOCK:BMAL1 heterodimer [1][2]. Resumption of CLOCK:BMAL1 activity occurs only when these repressors are degraded by regulators such as the serine/threonine casein kinases (CK1δ and CK1ϵ), which phosphorylate PER, initiating its ubiquitination. CRY turnover is also controlled by phosphorylation; the metabolic sensor 5′ adenosine monophosphate-activated protein kinase (AMPK) tags it for proteasome degradation by direct phosphorylation. Additional loops consisting of the nuclear receptor subfamily 1 D member 1 (NR1D1) also known as REV-ERα/β and the retinoic acid receptor-related orphan receptors (RORs) sustain this core transcriptional loop by transactivating or repressing Bmal1 [3][4][5]. An integral non-circadian loop intertwined with the core clock includes the circadian metabolite nicotinamide adenine dinucleotide (NAD
+
). The NAD
+
-dependent deacetylase sirtuin 1 (SIRT1) directly binds to the CLOCK:BMAL1 heterodimer, and thereby regulates the NAD
+
salvage pathway transcriptionally [6]. Together, these feedback loops mediate rhythmic expression of hundreds of clock-controlled genes (
). Importantly,
Bmal1 is the only gene in which single-gene knockout results in full loss of rhythmicity at the cellular and behavioral levels in a normal light–dark cycle [7,8], though double knockouts of
is the only gene in which single-gene knockout results in full loss of rhythmicity at the cellular and behavioral levels in a normal light–dark cycle [7][8], though double knockouts of
Cry1
and
Cry2 can result in complete arrhythmicity in constant darkness [9,10]. This underscores the robust and resilient, though highly intricate, nature of our internal clocks to maintaining time.
can result in complete arrhythmicity in constant darkness [9][10]. This underscores the robust and resilient, though highly intricate, nature of our internal clocks to maintaining time.
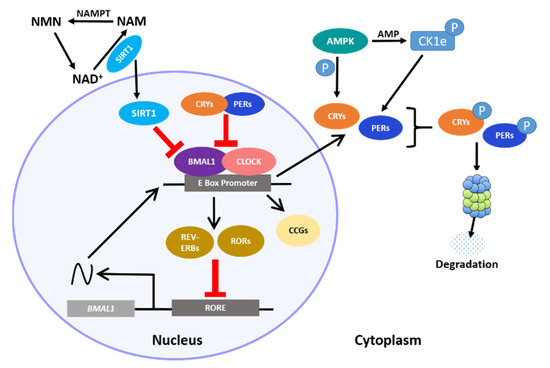
Interactions between the core clock and intracellular metabolism. The heterodimerization of BMAL1 and CLOCK proteins and subsequent binding to E-Box-containing regulatory elements leads to expression of the repressor PER and CRY proteins, the REV-ERBs and RORs, which initiate the auxiliary loop, and the core clock genes (CCGs) that drive numerous other intracellular rhythms. Cytoplasmic PER and CRY proteins are eventually tagged for degradation by AMPK. Rhythmic cellular metabolism, such as rhythmic NAD
abundance, participates in the core clock by direct regulation of clock-associated factors, such as the anti-aging-associated histone deacetylase protein, SIRT1.
Numerous epidemiological studies have shed light on the importance of rhythmicity on health. Over time, disruption of our 24 h cycle can lead to deleterious physiological outcomes, such as premature aging, and an increased risk for several diseases and disorders including obesity, cardiovascular disease, Alzheimer’s disease and other neurological diseases [11,12,13,14,15,16,17]. Epidemiological studies on night shift workers have revealed a type of desynchrony or “misalignment” of biological processes in individuals under shift work [18]. The circadian mechanisms driving daytime wakefulness find themselves in conflict with the homeostatic drive for sleep that accumulates as daytime progresses, leading to circadian perturbation [19,20,21]. Shift work and jet lag are not the only forms of circadian disruption; nutrient stress (a prominent disruptor of peripheral rhythms [22]), nocturnal light pollution, and mistimed food intake are additional examples of potent
Numerous epidemiological studies have shed light on the importance of rhythmicity on health. Over time, disruption of our 24 h cycle can lead to deleterious physiological outcomes, such as premature aging, and an increased risk for several diseases and disorders including obesity, cardiovascular disease, Alzheimer’s disease and other neurological diseases [11][12][13][14][15][16][17]. Epidemiological studies on night shift workers have revealed a type of desynchrony or “misalignment” of biological processes in individuals under shift work [18]. The circadian mechanisms driving daytime wakefulness find themselves in conflict with the homeostatic drive for sleep that accumulates as daytime progresses, leading to circadian perturbation [19][20][21]. Shift work and jet lag are not the only forms of circadian disruption; nutrient stress (a prominent disruptor of peripheral rhythms [22]), nocturnal light pollution, and mistimed food intake are additional examples of potent
zeitgebers (or “time-givers”) that alter the internal biological clock and its synchrony across tissues [23,24].
(or “time-givers”) that alter the internal biological clock and its synchrony across tissues [23][24].
Photic stimulation is the most powerful
zeitgeber for the brain’s clock. Light is directly received by retinal ganglion cells (ipRGCs), which contain the photopigment melanopsin. The ipRGCs depolarize independently from the rods and cones to relay light information to the SCN, a small region of the anterior hypothalamus with critical synchronizing capabilities. A combination of ablation studies in rats and monkeys, along with clinical psychiatric observations carried out in the mid-1900s first implicated a circadian clock in the hypothalamic region [25,26]. However, it was not until the discovery of the retinohypothalamic tract (RHT) in rats that the SCN was proven to be important for rhythmicity [27]. The SCN was identified in 1972 by two groups, who showed that electrolytic lesion of the SCN in rats resulted in the loss of locomotor and drinking rhythmicity [28,29]. Electrophysiological studies in rats demonstrated that SCN rhythmicity could be maintained for days in vitro following ex-plantation from the surrounding brain tissue [30]. Later studies also revealed that arrhythmicity in hamsters with SCN lesions could be restored when a fetal SCN was grafted onto the lesioned SCN in vivo [31]. Together, these studies underscore the robustness of the SCN and its requirement for circadian rhythms and behavior (reviewed in Herzog et al. 2017 [32]). The SCN coordinates the entire mammalian circadian system, through the complex regulation of electrical and hormonal signals that propagate throughout the brain and the periphery [33]. Though initially thought to be the dominant component of the 24 h biological clock in the mammalian system, genetic editing tools have revealed new roles of the circadian clock throughout various tissues of the body, where it controls processes as disparate as glucose sensitization, fluid balance, immune defense, lipid metabolism, and cell migration, among many others [34,35,36,37,38,39,40,41]. Moreover, when explanted from the body into culture, these tissue clocks maintain rhythms, indicating their own autonomy [42].
for the brain’s clock. Light is directly received by retinal ganglion cells (ipRGCs), which contain the photopigment melanopsin. The ipRGCs depolarize independently from the rods and cones to relay light information to the SCN, a small region of the anterior hypothalamus with critical synchronizing capabilities. A combination of ablation studies in rats and monkeys, along with clinical psychiatric observations carried out in the mid-1900s first implicated a circadian clock in the hypothalamic region [25][26]. However, it was not until the discovery of the retinohypothalamic tract (RHT) in rats that the SCN was proven to be important for rhythmicity [27]. The SCN was identified in 1972 by two groups, who showed that electrolytic lesion of the SCN in rats resulted in the loss of locomotor and drinking rhythmicity [28][29]. Electrophysiological studies in rats demonstrated that SCN rhythmicity could be maintained for days in vitro following ex-plantation from the surrounding brain tissue [30]. Later studies also revealed that arrhythmicity in hamsters with SCN lesions could be restored when a fetal SCN was grafted onto the lesioned SCN in vivo [31]. Together, these studies underscore the robustness of the SCN and its requirement for circadian rhythms and behavior (reviewed in Herzog et al. 2017 [32]). The SCN coordinates the entire mammalian circadian system, through the complex regulation of electrical and hormonal signals that propagate throughout the brain and the periphery [33]. Though initially thought to be the dominant component of the 24 h biological clock in the mammalian system, genetic editing tools have revealed new roles of the circadian clock throughout various tissues of the body, where it controls processes as disparate as glucose sensitization, fluid balance, immune defense, lipid metabolism, and cell migration, among many others [34][35][36][37][38][39][40][41]. Moreover, when explanted from the body into culture, these tissue clocks maintain rhythms, indicating their own autonomy [42].
2. Chronology of Clocks in the Hypothalamic Nuclei
Hypothalamic extra-SCN oscillators are now recognized to play integral roles in essential physiological functions such as eating, sleep–wake cycles, energy metabolism and thermoregulation [43,44,45,46]. One example of diurnal activity considered to be controlled independently of the SCN is food-anticipatory activity (FAA), which can persist in spite of SCN ablation [47]. As the name suggests, FAA involves increased activity in anticipation of an upcoming meal, which is particularly evident when daily feeding is restricted to a temporally restrictive time window. Rats express FAA in constant darkness (or “free-running” conditions) and even in the absence of a functional SCN [47]. These findings point to an elusive food-entrainable circadian oscillator (FEO) which is independent of the SCN [48].
Hypothalamic extra-SCN oscillators are now recognized to play integral roles in essential physiological functions such as eating, sleep–wake cycles, energy metabolism and thermoregulation [43][44][45][46]. One example of diurnal activity considered to be controlled independently of the SCN is food-anticipatory activity (FAA), which can persist in spite of SCN ablation [47]. As the name suggests, FAA involves increased activity in anticipation of an upcoming meal, which is particularly evident when daily feeding is restricted to a temporally restrictive time window. Rats express FAA in constant darkness (or “free-running” conditions) and even in the absence of a functional SCN [47]. These findings point to an elusive food-entrainable circadian oscillator (FEO) which is independent of the SCN [48].
To date, how these extra-SCN oscillators function relative to or in coordination with the SCN is still under investigation. The SCN has direct projections to various hypothalamic regions as well as non-hypothalamic regions such as the periventricular nucleus of the thalamus, the intergeniculate leaflet, the lateral septum and the periaqueductal gray [49]. However, the SCN predominantly innervates hypothalamic nuclei where dense projections to the subparaventricular zone (SPVZ) and the medial preoptic area (MPOA) have been observed [50,51,52]. Neural tracing studies have revealed that the dorsal medial hypothalamus (DMH), arcuate nucleus (ARC), lateral hypothalamus (LH), paraventricular nucleus (PVN), ventral lateral hypothalamus (VMH), and the ventral lateral preoptic area (VLPO) are also targets of SCN projections [50,53] (
To date, how these extra-SCN oscillators function relative to or in coordination with the SCN is still under investigation. The SCN has direct projections to various hypothalamic regions as well as non-hypothalamic regions such as the periventricular nucleus of the thalamus, the intergeniculate leaflet, the lateral septum and the periaqueductal gray [49]. However, the SCN predominantly innervates hypothalamic nuclei where dense projections to the subparaventricular zone (SPVZ) and the medial preoptic area (MPOA) have been observed [50][51][52]. Neural tracing studies have revealed that the dorsal medial hypothalamus (DMH), arcuate nucleus (ARC), lateral hypothalamus (LH), paraventricular nucleus (PVN), ventral lateral hypothalamus (VMH), and the ventral lateral preoptic area (VLPO) are also targets of SCN projections [50][53] (
Figure 2). Collectively, the ARC, VMH, LH and PVN are heavily involved with hunger and satiety as well as metabolic balance [54,55,56,57,58,59,60,61,62,63]. Moreover, neurons in the PVN are also responsible for endocrine regulation through hormone production and release [64,65]. The LH, MPOA, VLPO, DMH and SPVZ regulate sleep/wakefulness, locomotion, and thermoregulation [63,66,67,68,69]. Altogether, these hypothalamic nuclei maintain organism-wide energy balance.
). Collectively, the ARC, VMH, LH and PVN are heavily involved with hunger and satiety as well as metabolic balance [54][55][56][57][58][59][60][61][62][63]. Moreover, neurons in the PVN are also responsible for endocrine regulation through hormone production and release [64][65]. The LH, MPOA, VLPO, DMH and SPVZ regulate sleep/wakefulness, locomotion, and thermoregulation [63][66][67][68][69]. Altogether, these hypothalamic nuclei maintain organism-wide energy balance.

A circuit of hypothalamic oscillators. Numerous afferent and efferent projections characterize the hypothalamic landscape. The “master clock”, or the SCN, projects to the major hypothalamic nuclei while only a few nuclei project back to the SCN. Some regions, such as the DMH and ARC host an autonomous clock while other regions such as the PVN, LH and MPOA/VLPO are more heavily dependent on rhythmic innervations. Clock autonomy for the VMH and SPVZ has not been shown. An array of neural subpopulations in the hypothalamus are sensitive to hormones such as leptin, ghrelin, cholecystokinin (CCK), glucose and insulin, which cross the blood–brain barrier (BBB). These hypothalamic nuclei are often characterized by their inhibitory glutamatergic (blue), or their excitatory GABAergic (orange) projections. The gray dotted vertical lines denote the different regions of the hypothalamus. The general flow of information progresses from anterior to posterior, as indicated by the black arrow. Gastrin-Releasing Peptide; TRH = tyrosine hydroxylase; TRPM2 = transient receptor potential cation channel, subfamily M, member 2; VIP = vasoactive intestinal peptide.
Several years following the discovery of extra-SCN-driven circadian rhythms of melatonin release by the retina [70], 27 brain regions were examined for rhythmicity independent of the SCN [71]. Utilizing genetically modified rats harboring a
Period1
promoter-driven luciferase (
Per1-luc
) transgene, real-time bioluminescent recordings of isolated brain tissues cultured in vitro [71] revealed that 14 out of the 27 hypothalamic regions examined were able to maintain autonomous rhythms in
Per1
-
luc
expression [71]. The most robust rhythms were found in the olfactory bulb, the ARC, the pituitary gland and the PVN [71]. Though the SCN is able to maintain rhythmicity over very long periods of time in vitro, non-SCN tissue rhythms dampened much more quickly. This is thought to be due to the loss of synchronization provided by the SCN between individual cells of other hypothalamic nuclei [42]. Nevertheless, these data suggested a role for semi-autonomous extra-SCN oscillators not only in the hypothalamus, but other regions of the central nervous system (CNS) as well.
3. Circadian Regulation of Metabolism by Neuroendocrine Hormones
Metabolic homeostasis is predominantly synchronized by the hypothalamic clocks. However, reciprocal relationships between the hypothalamus and peripheral endocrine organs are also required (
Figure 5). For example, physiological fluctuations triggered by external stressors, such as changes in blood pressure or glucose levels, can influence SCN neuronal behavior [224]. The sympathetic and parasympathetic branches of the ANS feedback to hypothalamic areas, namely the PVN, which then may indirectly relay the information to the SCN. Furthermore, nutrients and hormones that cross the BBB will be sensed by the ARC-ME complex, and relayed to upstream hypothalamic areas such as the PVN. This is particularly important for the rhythmically fluctuating hunger and satiety hormones, such as leptin and ghrelin. Adipose-derived leptin activates NPY/AgRP and POMC neurons to suppress food intake and stimulate energy expenditure [225]. Leptin also stimulates fatty acid oxidation in skeletal muscle, promotes the uptake of glucose, and improves insulin sensitivity via central and peripheral mechanisms [226,227]. Expression of leptin receptors in POMC neurons of morbidly obese, diabetic and leptin receptor-deficient mice (
3). For example, physiological fluctuations triggered by external stressors, such as changes in blood pressure or glucose levels, can influence SCN neuronal behavior [72]. The sympathetic and parasympathetic branches of the ANS feedback to hypothalamic areas, namely the PVN, which then may indirectly relay the information to the SCN. Furthermore, nutrients and hormones that cross the BBB will be sensed by the ARC-ME complex, and relayed to upstream hypothalamic areas such as the PVN. This is particularly important for the rhythmically fluctuating hunger and satiety hormones, such as leptin and ghrelin. Adipose-derived leptin activates NPY/AgRP and POMC neurons to suppress food intake and stimulate energy expenditure [73]. Leptin also stimulates fatty acid oxidation in skeletal muscle, promotes the uptake of glucose, and improves insulin sensitivity via central and peripheral mechanisms [74][75]. Expression of leptin receptors in POMC neurons of morbidly obese, diabetic and leptin receptor-deficient mice (
Leprdb/db) results in a reduction in energy intake accompanied by normalized glucose levels and attenuated body weight gain [228]. Interestingly, leptin production in WAT is sufficient to drive diurnal oscillations in circulating leptin, independent of feeding. Adipose-specific ablation of
) results in a reduction in energy intake accompanied by normalized glucose levels and attenuated body weight gain [76]. Interestingly, leptin production in WAT is sufficient to drive diurnal oscillations in circulating leptin, independent of feeding. Adipose-specific ablation of
Bmal1 does alter the energy regulation of mice as reflected by reduced levels of triglyceride and polyunsaturated fatty acids circulating through the hypothalamus [190]. In addition, the SCN potentiates the response of ARC neurons to circulating leptin to maintain long-term homeostasis in energy balance [229].
does alter the energy regulation of mice as reflected by reduced levels of triglyceride and polyunsaturated fatty acids circulating through the hypothalamus [77]. In addition, the SCN potentiates the response of ARC neurons to circulating leptin to maintain long-term homeostasis in energy balance [78].
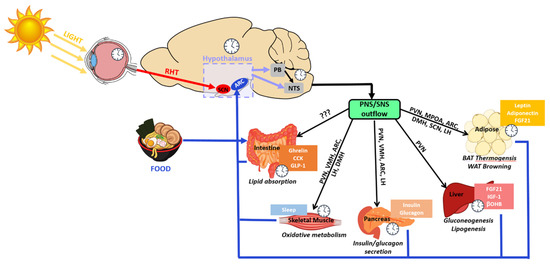
Hypothalamic-mediated circadian regulation of physiological functions. Powerful
such as light and food have extensive effects on the circadian activity of cellular and tissue functions. The light-sensitive ipRGCs in the retina send signals to the master clock, the SCN, via the RHT. Food consumption stimulates various metabolic and digestive mechanisms which produce a variety of molecular signals and hormones. Nutrient-sensitive neurons in the circadian-regulated hypothalamus pick up the fluctuations in nutrients and hormones and relay the information downstream to the PB and the NTS which regulate the function of tissue processes through the parasympathetic and the sympathetic nervous system (PNS/SNS). With the exception of the intestines, which currently have not been found to have direct innervation from any hypothalamic nuclei, many metabolic-regulating tissues have direct or indirect innervations from various hypothalamic nuclei.
3.1. Cortisol-Releasing Hormone (CRH)
A range of neuroendocrine hormones are produced by several hypothalamic nuclei and circulated by secretion into the third ventricle. This includes the well-known oscillatory glucocorticoid hormone, CORT, which is produced by PVN corticotrophin-releasing hormone (CRH) neurons and is critical in the hypothalamic–pituitary–adrenal (HPA) stress axis. Although typically associated with stress, CORT, has been shown to reflect variations in metabolic rate independent of psychological stress [230]. CORT circulation is highly rhythmic, returning to the brain through the ARC. ARC detection of CORT also follows a circadian pattern, dependent on target glucocorticoid and mineralocorticoid receptors in the ARC [231]. Prolonged elevation of glucocorticoid levels in mice results in overconsumption of food via inhibition of CRH-expressing neurons, lowering CORT levels and stimulating NPY expression [232,233]. Additional studies have revealed that both NPY and AgRP expression are differentially responsive to stress via direct innervation from PVN-CRH neurons to the ARC [234,235,236]. NPY neurons of the ARC innervate the PVN, resulting in NPY-mediated CORT production, food intake, and increased PVN activity [237,238]. NPY injection in the PVN of mice results in BAT thermogenesis and increased WAT lipoprotein lipase enzymatic activity [55]. Microarray analyses reveal that rhythmic NPY neurons in part control circadian transcriptional activity in the mouse liver [239]. These findings underscore a bidirectional relationship between the HPA axis and rhythmic ARC and PVN neurons, suggesting an important feedback loop whereby stress-induced chronodisruption influences metabolism and food intake. The recent identification of a neurocircuit whereby GABAergic SCN neurons project to CRH-PVN neurons which excite the OX-LH neurons stimulating wakefulness in mice [115] further highlights the diverse involvement of the CRH neurons and their CORT production in balancing homeostatic processes.A range of neuroendocrine hormones are produced by several hypothalamic nuclei and circulated by secretion into the third ventricle. This includes the well-known oscillatory glucocorticoid hormone, CORT, which is produced by PVN corticotrophin-releasing hormone (CRH) neurons and is critical in the hypothalamic–pituitary–adrenal (HPA) stress axis. Although typically associated with stress, CORT, has been shown to reflect variations in metabolic rate independent of psychological stress [79]. CORT circulation is highly rhythmic, returning to the brain through the ARC. ARC detection of CORT also follows a circadian pattern, dependent on target glucocorticoid and mineralocorticoid receptors in the ARC [80]. Prolonged elevation of glucocorticoid levels in mice results in overconsumption of food via inhibition of CRH-expressing neurons, lowering CORT levels and stimulating NPY expression [81][82]. Additional studies have revealed that both NPY and AgRP expression are differentially responsive to stress via direct innervation from PVN-CRH neurons to the ARC [83][84][85]. NPY neurons of the ARC innervate the PVN, resulting in NPY-mediated CORT production, food intake, and increased PVN activity [86][87]. NPY injection in the PVN of mice results in BAT thermogenesis and increased WAT lipoprotein lipase enzymatic activity [55]. Microarray analyses reveal that rhythmic NPY neurons in part control circadian transcriptional activity in the mouse liver [88]. These findings underscore a bidirectional relationship between the HPA axis and rhythmic ARC and PVN neurons, suggesting an important feedback loop whereby stress-induced chronodisruption influences metabolism and food intake. The recent identification of a neurocircuit whereby GABAergic SCN neurons project to CRH-PVN neurons which excite the OX-LH neurons stimulating wakefulness in mice [89] further highlights the diverse involvement of the CRH neurons and their CORT production in balancing homeostatic processes.
3.2. Melatonin
The hypothalamic-regulated hormone melatonin is synthesized in the pineal gland and is integral to sleep/wakefulness. Melatonin peaks during the dark phase in both diurnal and nocturnal organisms, whereas most hormones are expressed at opposite phases between nocturnal and diurnal organisms. This suggests that melatonin release is regulated by mechanisms upstream of the unknown biological diurnal/nocturnal switch. Control of melatonin release occurs via direct excitatory glutamatergic inputs from the SCN to the pineal gland. During the dark phase, direct GABAergic inhibitory signals from the SCN to the PVN inhibit the PVN to pineal gland projections [118,240]. Rhythmicity in melatonin provides essential regulatory control in metabolism; when melatonin synthesis is abolished by pinealectomy, glucose tolerance is impaired and blood glucose rhythmicity is completely lost under ad libitum feeding conditions [241,242]. Thus, the melatonin-mediated regulation of plasma glucose levels is controlled by a delicate balance of glutamatergic and GABAergic pre-autonomic hypothalamic inputs. The daily rise in plasma glucose is produced by inhibition of GABAergic activity at sympathetic pre-autonomic neurons of the PVN, which also increases hepatic glucose output [243]. Altogether, melatonin acts as a key integrator of circadian rhythms and energy metabolism through its effects on the hypothalamus and the periphery.The hypothalamic-regulated hormone melatonin is synthesized in the pineal gland and is integral to sleep/wakefulness. Melatonin peaks during the dark phase in both diurnal and nocturnal organisms, whereas most hormones are expressed at opposite phases between nocturnal and diurnal organisms. This suggests that melatonin release is regulated by mechanisms upstream of the unknown biological diurnal/nocturnal switch. Control of melatonin release occurs via direct excitatory glutamatergic inputs from the SCN to the pineal gland. During the dark phase, direct GABAergic inhibitory signals from the SCN to the PVN inhibit the PVN to pineal gland projections [90][91]. Rhythmicity in melatonin provides essential regulatory control in metabolism; when melatonin synthesis is abolished by pinealectomy, glucose tolerance is impaired and blood glucose rhythmicity is completely lost under ad libitum feeding conditions [92][93]. Thus, the melatonin-mediated regulation of plasma glucose levels is controlled by a delicate balance of glutamatergic and GABAergic pre-autonomic hypothalamic inputs. The daily rise in plasma glucose is produced by inhibition of GABAergic activity at sympathetic pre-autonomic neurons of the PVN, which also increases hepatic glucose output [94]. Altogether, melatonin acts as a key integrator of circadian rhythms and energy metabolism through its effects on the hypothalamus and the periphery.
3.3. Gut-Derived Polypeptides
Gut-derived polypeptides have also been implicated in circadian rhythmicity. Apart from the previously discussed GLP-1 and FGF21 hormones, other peripherally-derived hormones also modulate metabolism in a hypothalamus-dependent manner. The gastrin-releasing peptide (GRP) is a mediator of food intake and locomotor activity, and can induce light-like resetting of the SCN [244]. Recently, microbes residing in the gut have been shown to be highly relevant circadian factors in metabolism. Studies have demonstrated that certain microbial taxa and their secretions exhibit diurnal oscillations [245,246,247,248]. Additionally, the timing of food intake and chronodisruptions such as jet lag and shift work, can alter the abundance and functions of gut microbes [248,249]. Most interestingly, germ free mice which lack gut microbiota, actually display altered SCN and hepatic transcriptional rhythms, particularly in core clock genes and metabolic pathways [248]. While the gut microbe interaction with the clock machinery is still under investigation, these studies suggest an important relationship that may influence overall energy metabolism. Collectively, rhythmic cross-talk between the extra-SCN hypothalamus and the periphery is critical for metabolic homeostasis.
Gut-derived polypeptides have also been implicated in circadian rhythmicity. Apart from the previously discussed GLP-1 and FGF21 hormones, other peripherally-derived hormones also modulate metabolism in a hypothalamus-dependent manner. The gastrin-releasing peptide (GRP) is a mediator of food intake and locomotor activity, and can induce light-like resetting of the SCN [95]. Recently, microbes residing in the gut have been shown to be highly relevant circadian factors in metabolism. Studies have demonstrated that certain microbial taxa and their secretions exhibit diurnal oscillations [96][97][98][99]. Additionally, the timing of food intake and chronodisruptions such as jet lag and shift work, can alter the abundance and functions of gut microbes [99][100]. Most interestingly, germ free mice which lack gut microbiota, actually display altered SCN and hepatic transcriptional rhythms, particularly in core clock genes and metabolic pathways [99]. While the gut microbe interaction with the clock machinery is still under investigation, these studies suggest an important relationship that may influence overall energy metabolism. Collectively, rhythmic cross-talk between the extra-SCN hypothalamus and the periphery is critical for metabolic homeostasis.
References
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017, 18, 164–179.
- Partch, C.L.; Green, C.B.; Takahashi, J.S. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014, 24, 90–99.
- Crumbley, C.; Wang, Y.; Kojetin, D.J.; Burris, T.P. Characterization of the core mammalian clock component, NPAS2, as a REV-ERBα/RORα target gene. J. Biol. Chem. 2010, 285, 35386–35392.
- Gerhart-Hines, Z.; Feng, D.; Emmett, M.J.; Everett, L.J.; Loro, E.; Briggs, E.R.; Bugge, A.; Hou, C.; Ferrara, C.; Seale, P.; et al. The nuclear receptor Rev-erbα controls circadian thermogenic plasticity. Nature 2013, 503, 410–413.
- Kornmann, B.; Schaad, O.; Bujard, H.; Takahashi, J.S.; Schibler, U. System-driven and oscillator-dependent circadian transcription in mice with a conditionally active liver clock. PLoS Biol. 2007, 5, e34.
- Nakahata, Y.; Sahar, S.; Astarita, G.; Kaluzova, M.; Sassone-Corsi, P. Circadian Control of the NAD+ Salvage Pathway by CLOCK-SIRT1. Science 2009, 324, 654–657.
- Bunger, M.K.; Wilsbacher, L.D.; Moran, S.M.; Clendenin, C.; Radcliffe, L.A.; Hogenesch, J.B.; Simon, M.C.; Takahashi, J.S.; Bradfield, C.A. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 2000, 103, 1009–1017.
- Baggs, J.E.; Price, T.S.; Ditacchio, L.; Panda, S.; Fitzgerald, G.A.; Hogenesch, J.B. Network features of the mammalian circadian clock. PLoS Biol. 2009, 7, e1000052.
- Van Der Horst, G.T.J.; Muijtjens, M.; Kobayashi, K.; Takano, R.; Kanno, S.I.; Takao, M.; De Wit, J.; Verkerk, A.; Eker, A.P.M.; Van Leenen, D.; et al. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature 1999, 398, 627–630.
- Bae, K.; Jin, X.; Maywood, E.S.; Hastings, M.H.; Reppert, S.M.; Weaver, D.R. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron 2001.
- Musiek, E.S.; Xiong, D.D.; Holtzman, D.M. Sleep, circadian rhythms, and the pathogenesis of Alzheimer disease. Exp. Mol. Med. 2015, 47, e148.
- Bokenberger, K.; Sjölander, A.; Dahl Aslan, A.K.; Karlsson, I.K.; Åkerstedt, T.; Pedersen, N.L. Shift work and risk of incident dementia: A study of two population-based cohorts. Eur. J. Epidemiol. 2018, 33, 977–987.
- van der Vinne, V.; Martin Burgos, B.; Harrington, M.E.; Weaver, D.R. Deconstructing circadian disruption: Assessing the contribution of reduced peripheral oscillator amplitude on obesity and glucose intolerance in mice. J. Pineal Res. 2020, 69, 1–12.
- Parsons, M.J.; Moffitt, T.E.; Gregory, A.M.; Goldman-Mellor, S.; Nolan, P.M.; Poulton, R.; Caspi, A. Social jetlag, obesity and metabolic disorder: Investigation in a cohort study. Int. J. Obes. 2015, 39, 842–848.
- Cho, K.; Ennaceur, A.; Cole, J.C.; Suh, C.K. Chronic jet lag produces cognitive deficits. J. Neurosci. 2000, 20, 1–5.
- Reutrakul, S.; Knutson, K.L. Consequences of Circadian Disruption on Cardiometabolic Health. Sleep Med. Clin. 2015, 10, 455–467.
- Shi, S.Q.; Ansari, T.S.; McGuinness, O.P.; Wasserman, D.H.; Johnson, C.H. Circadian disruption leads to insulin resistance and obesity. Curr. Biol. 2013, 23, 372–381.
- James, S.M.; Honn, K.A.; Gaddameedhi, S.; Van Dongen, H.P.A. Shift Work: Disrupted Circadian Rhythms and Sleep—Implications for Health and Well-being. Curr. Sleep Med. Reports 2017, 3, 104–112.
- Borbely, A.A. A two process model of sleep regulation. Hum. Neurobiol. 1982, 1, 195–204.
- Daan, S.; Beersma, D.G.M.; Borbely, A.A. Timing of human sleep: Recovery process gated by a circadian pacemaker. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1984, 15.
- Dijk, D.J.; Czeisler, C.A. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci. Lett. 1994, 166, 63–68.
- Kohsaka, A.; Laposky, A.D.; Ramsey, K.M.; Estrada, C.; Joshu, C.; Kobayashi, Y.; Turek, F.W.; Bass, J. High-Fat Diet Disrupts Behavioral and Molecular Circadian Rhythms in Mice. Cell Metab. 2007, 6, 414–421.
- Navara, K.J.; Nelson, R.J. The dark side of light at night: Physiological, epidemiological, and ecological consequences. J. Pineal Res. 2007, 43, 215–224.
- Kolbe, I.; Leinweber, B.; Brandenburger, M.; Oster, H. Circadian clock network desynchrony promotes weight gain and alters glucose homeostasis in mice. Mol. Metab. 2019, 30, 140–151.
- Richter, C.P.; Hawkes, C.D. Increased Spontaneous activity and food intake produced in rats by removal of the frontal poles of the brain by. J. Neurol. Psychiatry 1939, 2, 231.
- Richter, C.P. Biological clocks in medicine and psychiatry: Shock-phase hypothesis. Proc. Natl. Acad. Sci. USA 1960, 46, 1506–1530.
- Moore, R.Y.; Lenn, N.J. A Retinohypothalamic Projection in the Rat. J. Comp. Neurol. 1972, 146, 1–14.
- Stephan, F.K.; Zucker, I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc. Natl. Acad. Sci. USA 1972, 69, 1583–1586.
- Moore, R.Y.; Eichler, V.B. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972, 42, 201–206.
- Kawamura, H. Hypothalamic “Island” Containing the Suprachiasmatic Nucleus. Neurobiology 1979, 76, 5962–5966.
- Ralph, M.R.; Foster, R.G.; Davis, F.C. Transplanted Suprachiasmatic Nucleus Deternines Circadian Period. Science 1990, 247, 975–978.
- Herzog, E.D.; Hermanstyne, T.; Smyllie, N.J.; Hastings, M.H. Regulating the suprachiasmatic nucleus (SCN) circadian clockwork: Interplay between cell- autonomous and circuit-level mechanisms. Cold Spring Harb. Perspect. Biol. 2017, 9.
- Hastings, M.H.; Maywood, E.S.; Brancaccio, M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat. Rev. Neurosci. 2018, 19, 453–469.
- Allaman-Pillet, N.; Roduit, R.; Oberson, A.; Abdelli, S.; Ruiz, J.; Beckmann, J.S.; Schorderet, D.F.; Bonny, C. Circadian regulation of islet genes involved in insulin production and secretion. Mol. Cell. Endocrinol. 2004, 226, 59–66.
- Keller, M.; Mazuch, J.; Abraham, U.; Eom, G.D.; Herzog, E.D.; Volk, H.D.; Kramer, A.; Maier, B. A circadian clock in macrophages controls inflammatory immune responses. Proc. Natl. Acad. Sci. USA 2009, 106, 21407–21412.
- Schmitt & Segert NIH Public Access. Bone 2008, 23, 1–7.
- Shostak, A.; Meyer-Kovac, J.; Oster, H. Circadian regulation of lipid mobilization in white adipose tissues. Diabetes 2013, 62, 2195–2203.
- Zhou, B.; Zhang, Y.; Zhang, F.; Xia, Y.; Liu, J.; Huang, R.; Wang, Y.; Hu, Y.; Wu, J.; Dai, C.; et al. CLOCK/BMAL1 regulates circadian change of mouse hepatic insulin sensitivity by SIRT1. Hepatology 2014, 59, 2196–2206.
- Carrasco-Benso, M.P.; Rivero-Gutierrez, B.; Lopez-Minguez, J.; Anzola, A.; Diez-Noguera, A.; Madrid, J.A.; Lujan, J.A.; Martínez-Augustin, O.; Scheer, F.A.J.L.; Garaulet, M. Human adipose tissue expresses intrinsic circadian rhythm in insulin sensitivity. FASEB J. 2016, 30, 3117–3123.
- Boucher, H.; Vanneaux, V.; Domet, T.; Parouchev, A.; Larghero, J. Circadian clock genes modulate human bone marrow mesenchymal stem cell differentiation, migration and cell cycle. PLoS ONE 2016, 11, e0146674.
- Zhang, Y.; Zhang, W.; Liu, C. Integration of peripheral circadian clock and energy metabolism in metabolic tissues. J. Mol. Cell Biol. 2020, 12, 481–485.
- Yoo, S.; Yamazaki, S.; Lowrey, P.L.; Shimomura, K.; Ko, C.H.; Buhr, E.D.; Siepka, S.M.; Hong, H.; Oh, W.J.; Yoo, O.J.; et al. PERIOD2: LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Natl. Acad. Sci. USA 2004, 101, 5339–5346.
- Cedernaes, J.; Waldeck, N.; Bass, J. Neurogenetic basis for circadian regulation of metabolism by the hypothalamus. Genes Dev. 2019, 33, 1136–1158.
- Greco, C.M.; Corsi, P.S. Circadian blueprint of metabolic pathways in the brain. Nat. Rev. Neurosci. 2019, 20, 71–82.
- Begemann, K.; Neumann, A.M.; Oster, H. Regulation and function of extra-SCN circadian oscillators in the brain. Acta Physiol. 2020, 229, 1–14.
- Paul, J.R.; Davis, J.A.; Goode, L.K.; Becker, B.K.; Fusilier, A.; Meador-Woodruff, A.; Gamble, K.L. Circadian regulation of membrane physiology in neural oscillators throughout the brain. Eur. J. Neurosci. 2020, 51, 109–138.
- Stephan, F.K.; Zucker, I. Circadian Rhythms. Science 1972, 69, 1583–1586.
- Stephan, F.K. The “other” circadian system: Food as a zeitgeber. J. Biol. Rhythms 2002, 17, 284–292.
- Morin, L.P. The circadian visual system. Brain Res. Rev. 1994, 19, 102–127.
- Abrahamson, E.E.; Moore, R.Y. Suprachiasmatic nucleus in the mouse: Retinal innervation, intrinsic organization and efferent projections. Brain Res. 2001, 916, 172–191.
- Swanson, L.W.; Cowan, W.M. The efferent connections of the suprachiasmatic nucleus of the hypothalamus. J. Comp. Neurol. 1975.
- Berk, M.L.; Finkelstein, J.A. An autoradiographic determination of the efferent projections of the suprachiasmatic nucleus of the hypothalamus. Brain Res. 1981.
- Kriegsfeld, L.J.; Leak, R.K.; Yackulic, C.B.; Sauter, J.L.E.; Silver, R.A.E. Organization of Suprachiasmatic Nucleus Projections in Syrian Hamsters (Mesocricetus auratus): An Anterograde and Retrograde Analysis. J. Comp. Neurol. 2004, 379, 361–379.
- Melnick, I.; Krishtal, O.A.; Colmers, W.F. Integration of energy homeostasis and stress by parvocellular neurons in rat hypothalamic paraventricular nucleus. J. Physiol. 2020.
- Billington, C.J.; Briggs, J.E.; Harker, S.; Grace, M.; Levine, A.S. Neuropeptide Y in hypothalamic paraventricular nucleus: A center coordinating energy metabolism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1994, 266.
- Satoh, N.; Ogawa, Y.; Katsuura, G.; Hayase, M.; Tsuji, T.; Imagawa, K.; Yoshimasa, Y.; Nishi, S.; Hosoda, K.; Nakao, K. The arcuate nucleus as a primary site of satiety effect of leptin in rats. Neurosci. Lett. 1997, 224, 149–152.
- Padilla, S.L.; Perez, J.G.; Ben-Hamo, M.; Johnson, C.W.; Sanchez, R.E.A.; Bussi, I.L.; Palmiter, R.D.; de la Iglesia, H.O. Kisspeptin Neurons in the Arcuate Nucleus of the Hypothalamus Orchestrate Circadian Rhythms and Metabolism. Curr. Biol. 2019, 29, 592–604.
- Kim, K.W.; Donato, J.; Berglund, E.D.; Choi, Y.H.; Kohno, D.; Elias, C.F.; DePinho, R.A.; Elmquist, J.K. FOXO1 in the ventromedial hypothalamus regulates energy balance. J. Clin. Investig. 2012.
- Çakir, I.; Perello, M.; Lansari, O.; Messier, N.J.; Vaslet, C.A.; Nillni, E.A. Hypothalamic Sirt1 regulates food intake in a rodent model system. PLoS ONE 2009.
- Leibowitz, S.F. Reciprocal hunger-regulating circuits involving alpha- and beta-adrenergic receptors located, respectively, in the ventromedial and lateral hypothalamus. Proc. Natl. Acad. Sci. USA 1970, 67, 1063–1070.
- Berthoud, H.R.; Münzberg, H. The lateral hypothalamus as integrator of metabolic and environmental needs: From electrical self-stimulation to opto-genetics. Physiol. Behav. 2011.
- Williams, G.; Harrold, J.A.; Cutler, D.J. The hypothalamus and the regulation of energy homeostasis: Lifting the lid on a black box. Proc. Nutr. Soc. 2000, 59, 385–396.
- Arrigoni, E.; Chee, M.J.S.; Fuller, P.M. To eat or to sleep: That is a lateral hypothalamic question. Neuropharmacology 2019, 154, 34–49.
- Nader, N.; Chrousos, G.P.; Kino, T. Interactions of the circadian CLOCK system and the HPA axis. Trends Endocrinol. Metab. 2010.
- Swanson, L.W.; Kuypers, H.G.J.M. The paraventricular nucleus of the hypothalamus: Cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J. Comp. Neurol. 1980, 194, 555–570.
- Chou, T.C.; Scammell, T.E.; Gooley, J.J.; Gaus, S.E.; Saper, C.B.; Lu, J. Critical Role of Dorsomedial Hypothalamic Nucleus in a Wide Range of Behavioral Circadian Rhythms. J. Neurosci. 2003, 23, 10691–10702.
- Lu, J.; Zhang, Y.H.; Chou, T.C.; Gaus, S.E.; Elmquist, J.K.; Shiromani, R.; Saper, C.B. Contrasting effects of ibotenate lesions of the paraventricular nucleus and subparaventricular zone on sleep-wake cycle and temperature regulation. J. Neurosci. 2001, 21, 4864–4874.
- Asala, S.A.; Okano, Y.; Honda, K.; Inoué, S. Effects of medial preoptic area lesions on sleep and wakefulness in unrestrained rats. Neurosci. Lett. 1990, 114, 300–304.
- Mondino, A.A.; Hambrecht-wiedbusch, V.; Li, D.; York, A.K. Glutamatergic neurons in the preoptic hypothalamus promote wakefulness, destabilize NREM sleep, suppress REM sleep, and regulate cortical dynamics. bioRxiv 2020.
- Tosini, G.; Menaker, M. Circadian Rhythms in Cultured Mammalian Retina. Science 1996, 272, 419–422.
- Abe, M.; Herzog, E.D.; Yamazaki, S.; Straume, M.; Tei, H.; Sakaki, Y.; Menaker, M.; Block, G.D. Circadian rhythms in isolated brain regions. J. Neurosci. 2002, 22, 350–356.
- Kahn, B.B.; Flier, J.S. Obesity and insulin resistance. J. Clin. Investig. 2000, 106, 473–481.
- Huo, L.; Gamber, K.; Greeley, S.; Silva, J.; Huntoon, N.; Leng, X.H.; Bjørbæk, C. Leptin-Dependent Control of Glucose Balance and Locomotor Activity by POMC Neurons. Cell Metab. 2009, 9, 537–547.
- Kettner, N.M.; Mayo, S.A.; Hua, J.; Lee, C.; Moore, D.D.; Fu, L. Circadian dysfunction induces leptin resistance in mice. Cell Metab. 2015, 22, 448–459.
- Paschos, G.K.; Ibrahim, S.; Song, W.L.; Kunieda, T.; Grant, G.; Reyes, T.M.; Bradfield, C.A.; Vaughan, C.H.; Eiden, M.; Masoodi, M.; et al. Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat. Med. 2012.
- Jimeno, B.; Hau, M.; Verhulst, S. Corticosterone levels reflect variation in metabolic rate, independent of ‘stress’. Sci. Rep. 2018, 8, 1–8.
- Gold, R.M. Hypothalamic obesity: The myth of the ventromedial nucleus. Science 1973.
- Leon-Mercado, L.; Chao, D.H.M.; del Carmen Basualdo, M.; Kawata, M.; Escobar, C.; Buijs, R.M. The arcuate nucleus: A site of fast negative feedback for corticosterone secretion in male rats. eNeuro 2017, 4, 1–14.
- Cavagnini, F.; Croci, M.; Putignano, P.; Petroni, M.L.; Invitti, C. Glucocorticoids and neuroendocrine function. Int. J. Obes. 2000.
- Kuperman, Y.; Weiss, M.; Dine, J.; Staikin, K.; Golani, O.; Ramot, A.; Nahum, T.; Kühne, C.; Shemesh, Y.; Wurst, W.; et al. CRFR1 in AgRP Neurons Modulates Sympathetic Nervous System Activity to Adapt to Cold Stress and Fasting. Cell Metab. 2016, 23, 1185–1199.
- Sefton, C.; Harno, E.; Davies, A.; Small, H.; Allen, T.J.; Wray, J.R.; Lawrence, C.B.; Coll, A.P.; White, A. Elevated hypothalamic glucocorticoid levels are associated with obesity and hyperphagia in male mice. Endocrinology 2016, 157, 4257–4265.
- Kas, M.J.H.; Bruijnzeel, A.W.; Haanstra, J.R.; Wiegant, V.M.; Adan, R.A.H. Differential regulation of agouti-related protein and neuropeptide Y in hypothalamic neurons following a stressful event. J. Mol. Endocrinol. 2005.
- Dimitrov, E.L.; DeJoseph, M.R.; Brownfield, M.S.; Urban, J.H. Involvement of neuropeptide Y Y1 receptors in the regulation of neuroendocrine corticotropin-releasing hormone neuronal activity. Endocrinology 2007, 148, 3666–3673.
- Stanley, B.G.; Leibowitz, S.F. Neuropeptide Y injected in the paraventricular hypothalamus: A powerful stimulant of feeding behavior. Proc. Natl. Acad. Sci. USA 1985.
- Erion, R.; King, A.N.; Wu, G.; Hogenesch, J.B.; Sehgal, A. Neural clocks and neuropeptide F/Y regulate circadian gene expression in a peripheral metabolic tissue. Elife 2016, 5, 1–21.
- Borjigin, J.; Samantha Zhang, L.; Calinescu, A.A. Circadian regulation of pineal gland rhythmicity. Mol. Cell. Endocrinol. 2012, 349, 13–19.
- Forrestel, A.C.; Miedlich, S.U.; Yurcheshen, M.; Wittlin, S.D.; Sellix, M.T. Chronomedicine and type 2 diabetes: Shining some light on melatonin. Diabetologia 2017, 60, 808–822.
- La Fleur, S.E.; Kalsbeek, A.; Wortel, J.; Van Der Vliet, J.; Buijs, R.M. Role for the pineal and melatonin in glucose homeostasis: Pinealectomy increases night- time glucose concentrations. J. Neuroendocrinol. 2001.
- Ono, D.; Mukai, Y.; Hung, C.J.; Chowdhury, S.; Sugiyama, T.; Yamanaka, A. Neuron The mammalian circadian pacemaker regulates wakefulness via CRF neurons in the paraventricular nucleus of the hypothalamus. Sci. Adv. 2020, 6, eabd0384.
- Kalsbeek, A.; Palm, I.F.; La Fleur, S.E.; Scheer, F.A.J.L.; Perreau-Lenz, S.; Ruiter, M.; Kreier, F.; Cailotto, C.; Buijs, R.M. SCN outputs and the hypothalamic balance of life. J. Biol. Rhythms 2006, 21, 458–469.
- Kalsbeek, A.; La Fleur, S.; Van Heijningen, C.; Buijs, R.M. Suprachiasmatic GABAergic inputs to the paraventricular nucleus control plasma glucose concentrations in the rat via sympathetic innervation of the liver. J. Neurosci. 2004, 24, 7604–7613.
- Gamble, K.L.; Allen, G.C.; Zhou, T.; McMahon, D.G. Gastrin-releasing peptide mediates light-like resetting of the suprachiasmatic nucleus circadian pacemaker through cAMP response element-binding protein and Per1 activation. J. Neurosci. 2007, 27, 12078–12087.
- Alvarez, Y.; Glotfelty, L.G.; Blank, N.; Dohnalová, L.; Thaiss, C.A. The Microbiome as a Circadian Coordinator of Metabolism. Endocrinology 2020, 161, 1–9.
- Thaiss, C.A.; Levy, M.; Korem, T.; Dohnalová, L.; Shapiro, H.; Jaitin, D.A.; David, E.; Winter, D.R.; Gury-BenAri, M.; Tatirovsky, E.; et al. Microbiota Diurnal Rhythmicity Programs Host Transcriptome Oscillations. Cell 2016.
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler, A.C.; Abramson, L.; Katz, M.N.; Korem, T.; Zmora, N.; et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 2014.
- Leone, V.; Gibbons, S.M.; Martinez, K.; Hutchison, A.L.; Huang, E.Y.; Cham, C.M.; Pierre, J.F.; Heneghan, A.F.; Nadimpalli, A.; Hubert, N.; et al. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe 2015.
- Liang, X.; Bushman, F.D.; FitzGerald, G.A. Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proc. Natl. Acad. Sci. USA 2015.
- Turek, F.W.; Joshu, C.; Kohsaka, A.; Lin, E.; Ivanova, G.; McDearmon, E.; Laposky, A.; Losee-Olson, S.; Easton, A.; Jensen, D.R.; et al. Obesity and metabolic syndrome in circadian Clock mutant nice. Science 2005, 308, 1043–1045.
- Yang, S.; Liu, A.; Weidenhammer, A.; Cooksey, R.C.; McClain, D.; Kim, M.K.; Aguilera, G.; Abel, E.D.; Chung, J.H. The role of mPer2 clock gene in glucocorticoid and feeding rhythms. Endocrinology 2009.
- Patel, S.R.; Hu, F.B. Short sleep duration and weight gain: A systematic review. Obesity 2008, 16, 643–653.
