Natural Killer (NK) cells are innate immune cells with the unique ability to recognize and kill virus-infected and cancer cells without prior immune sensitization. Radiotherapy is an anti-cancer strategy based on the administration of ionizing radiation, which induces DNA damage and cell death, and that is currently included in more than 50% of all anti-cancer treatments. Radiotherapy was found to directly impair NK cell viability and activity in a dose-dependent manner while modulating tumor cell sensitivity to NK cell-mediated cytotoxicity and the TME, potentially both promoting and impairing NK cell function, depending on dose and tumor heterogeneity, suggesting that combining radiotherapy with strategies to maintain NK cell viability and activity could be beneficial.
- NK cells
- radiotherapy
1. Introduction
Natural killer (NK) cells are large granular lymphocytes, part of the innate immune system, and are characterized by the expression of CD56, the absence of CD3 [1], and the ability to kill virus-infected and tumor cells without prior immune sensitization [2]. Classically, NK cells can be divided into two main subsets with distinct properties: the CD56dim effector NK cells with high cytotoxic capacity and the CD56bright NK cells, which exert mainly a regulatory function [3]. NK cell activity is controlled by a balance of inhibitory and activating receptors (Figure 1) [1]. Killer cell immunoglobulin-like receptors (KIR) and the natural killer receptor (NKG) 2A are examples of inhibitory receptors that are important to suppress non-specific cytotoxic activity and killing of healthy cells. They bind to multiple human leukocyte antigens (HLA), which can be downregulated by tumor and virus-infected cells to escape T cell recognition leading to increased NK cell recognition. In addition, NK cells can express other inhibitory receptors recognizing non-MHC molecules, such as the Lectin-like Transcript-1 (LLT-1), which binds to NKR-P1A. The activating receptors, such as natural killer group 2 member (NKG2)D/C/E, and natural cytotoxicity receptors (NCRs), such as NKp30, NKp46, and NKp44, recognize specific ligands, like MHC class I polypeptide–related sequence A/B (MICA/B), UL16 binding protein 1-6 (ULBP1-6), and heparan sulfate proteoglycan (HSPG), that are overexpressed by infected and malignant cells [1][2]. When the balance of these receptors is skewed towards activation, either due to a lack of inhibitory signals or the dominance of activating signals, NK cells are triggered to release cytotoxic granules and cytotoxic effector proteins like granzymes and perforin in order to kill the target cell [4][5]. NK cells can also induce apoptosis via Fas ligand and tumor necrosis factor (TNF)–related apoptosis-inducing ligand (TRAIL), which bind to the Fas receptor and the death receptor 5 (DR5) expressed on target cells [4]. Moreover, thanks to the expression of CD16 (Fc receptor: FcRγIII), NK cells can also kill through antibody-dependent cell-mediated cytotoxicity (ADCC) [6].
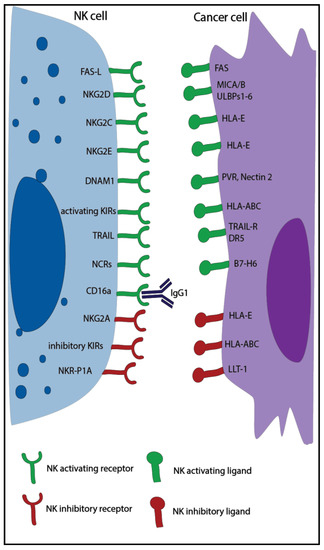
Overview of major natural killer (NK) cell activating and inhibitory receptors and their corresponding ligands expressed on tumor cells. KIR: killer cell immunoglobulin-like receptors; NKG: natural killer receptor; TRAIL: tumor necrosis factor-related apoptosis-inducing ligand; NCR: natural cytotoxicity receptors; HLA: human leukocyte antigen; MICA: MHC class I polypeptide–related sequence; PVR: poliovirus receptor; DR5: death receptor 5; DNAM1: DNAX Accessory Molecule-1, ULBP1-6: UL16 binding protein 1-6, LLT-1: lectin-like Transcript-1.
Cancer is a leading cause of death worldwide, and the number of new patients diagnosed with cancer is still rising globally [7]. In Europe, 20% of deaths are caused by cancer [8]. Although multiple therapeutic approaches are currently available, cancer remains a clinical challenge, and therefore new insights into this disease are necessary. NK cells are known to play a pivotal role in cancer. Patients with higher NK cell activity were found to have a better prognosis [9][10], and thanks to their ability to kill circulating cancer stem cells, which have high metastatic potential, NK cells play an essential role in the prevention of metastasis [11]. While the role of NK cells in monoclonal antibody-based therapies is well established [12], less is known on the function that NK cells have in other anti-cancer therapies.
2. Radiotherapy
Radiotherapy is an anti-cancer strategy based on the administration of ionizing radiation, which induces DNA damage and cell death, and that is currently included in more than 50% of all anti-cancer treatments [13][14]. Radiotherapy affects NK cells both directly and indirectly (Figure 2).

Direct and indirect effects of radiotherapy, chemotherapy, and protein kinase inhibitors on NK cell activity. Radiotherapy (
) and chemotherapy (
) cause cell damage, often leading to NK cell impairment, whereas protein kinase inhibitors (
) target specific signaling pathways resulting in either increased or decreased NK cell activity depending on the pathway involved. These treatments can also induce the modulation of various NK cell ligands on tumor cells and the release of damage-associated molecular patterns (DAMPs), indirectly affecting NK cell functions. Red arrows: inhibitory effects; green arrows: stimulatory effects; blue arrows: indirect effects, modulating the tumor cell’s susceptibility to NK-mediated cytolysis. ULBP1-6: UL16 binding protein 1-6; MICA: MHC class I polypeptide–related sequence; TRAIL: tumor necrosis factor-related apoptosis-inducing ligand; HLA: human leukocyte antigen; PD-(L)1: programmed cell death protein (ligand) 1, NKG: natural killer receptor; NCR: natural cytotoxicity receptors; BRAF: B-rapidly accelerated fibrosarcoma; GSK-3β: Glycogen synthase kinase-3β; JAK: Janus kinase.
The viability of ex vivo irradiated NK cells from healthy donors was shown to be reduced, and this decrease was directly correlated with a higher single radiation dose and the length of the post-irradiation measurement interval [15][16][17]. For instance, the mean percentage of dead NK cells after the administration of 1 Gy ranged from 1.3% to 20.7% after 2 and 42 h, respectively, whereas at the same time points, a dose of 10 Gy induced the death of 2.7% to 67.5% NK cells [15]. At low radiation doses (<0.1 Gy), ex vivo irradiated NK cells from healthy donors actually demonstrated higher levels of cytotoxicity compared to non-irradiated NK cells. Moreover, higher expression of TNFα and interferon-γ (IFNγ) was observed. Interestingly, the addition of a specific P38 inhibitor hampered the positive effect of low dose radiation on NK cell cytotoxicity, suggesting that the p38-mitogen-activated protein kinase (MAPK) pathway might mediate this effect [18]. In another study, occasionally higher NK cell cytotoxicity was found when ex vivo NK cells from healthy donors were irradiated with a single dose between 1–10 Gy compared to non-irradiated cells [15]. In addition, the administration of a total dose of 10 Gy in two fractions was observed to enhance ex vivo healthy donor NK cell cytotoxicity compared to the non-fractionated dose [17]. In contrast, a reduction in cytotoxicity was reported when ex vivo isolated NK cells from healthy donors were treated with higher radiation doses (>20 Gy) [15][17].
Multiple studies focusing on patients with cancer undergoing radiotherapy also unveiled reductions in the absolute number of various peripheral blood (PB) lymphocyte subsets, including NK cells [19][20][21][22][23][24][25], and impaired NK cell activity compared to pre-treatment levels [26][27], suggesting that radiotherapy directly decreases both NK cell viability and function in a dose-dependent manner.
The indirect effects of radiotherapy on NK cells can be divided into three categories: the modulation of activating and inhibitory NK ligands, the release of damage-associated molecular patterns (DAMPs), and the enhancement of NK cell migration to the tumor. Upon radiotherapy, many cell types, including tumor cells, modulate the expression of NK cell ligands with a crucial impact on the sensitization to NK cell responses. Cancer cells from various solid tumor types were discovered to upregulate MICA/B and ULPB1–3 [28][29][30][31], whereas they downregulated the KIR2D ligands HLA-ABC and HLA-G [32][33][34][35], suggesting a higher sensitivity to NK cell-mediated cytotoxicity. Moreover, multiple irradiated cancer cell lines showed an increased expression of the intracellular adhesion molecule 1 (ICAM1), which was described to enhance NK cell-mediated killing by increasing cell-to-cell adhesion, and the Fas receptor, possibly indicating higher susceptibility to NK cell-mediated apoptosis [32][33][36]. Of note, also cancer stem cells (CSC), which represent a small radio-resistant population, were found not only to upregulate the Fas receptor in an irradiation dose-dependent manner but also to upregulate MICA/B, suggesting higher sensitization to NK cell killing [37]. On the other hand, other irradiated cancer cell lines demonstrated to be more resistant to NK cell cytotoxicity by the downregulation of MICA/B, ULPB 1-3, or the upregulation of HLA-ABC [33][38]. It is important to note that different tumor cell lines were used to analyze these effects and that the discrepancies in the responses could be due to cell line specific properties. Indeed, a study analyzing expression levels of various proteins related to NK cell sensitivity (e.g., of Fas, HLA-ABC) on human colon, lung, and prostate cancer cell lines upon irradiation found heterogeneous responses [33]. Moreover, variation in the expression of NKG2D ligands (NKG2D-L; e.g., MICA/B, ULBP1-3) might be due to the upregulation of matrix-bound metalloproteinases (MMPs) by cancer cells, which can shed NKG2D-L from the tumor cell surface leading to decreased membrane expression, consequently reducing NK cell recognition and activation [31].
Radiotherapy can also induce the release of DAMPs by tumor cells, such as heat shock proteins (Hsp), which are a family of stress-inducible factors with anti-apoptotic function regularly expressed by tumor cells [39]. Higher levels of Hsp70 are produced in response to cellular stress, which can be caused by radiotherapy [40][41]. In addition to the intracellular anti-apoptotic function, the release of Hps70, or its expression on the cell surface, can function as a DAMP triggering anti-tumor immune responses. In particular, membrane-bound Hsp70 (mHsp70) can elicit NK cell activation and tumor cell killing through binding to NKG2A/C/E and the co-receptor CD94 [42][43]. However, the expression of HLA-E by tumor cells can hinder this mechanism, significantly reducing Hsp70-dependent activation [44]. Radiotherapy and genotoxic stress can also induce the release of other DAMPs such as adenosine triphosphate (ATP), which can be bound and processed into adenosine (ADO) by multiple cells in the tumor microenvironment (TME) (e.g., tumor cells, regulatory T cells (Treg), and CD8+ T cells) through their expression of CD39 and/or CD73. ADO is a highly immunosuppressive factor that impairs the function of multiple immune cells through the binding with its receptor, which is also expressed by NK cells [45][46].
Finally, radiotherapy enhanced NK cell migration to tumor cells in vitro. Multiple irradiated breast cancer cell lines were shown to increase their in vitro production of CXCL16, a CXCR6 ligand, leading to higher NK cell trans-well migration [47]. In conclusion, radiotherapy directly impaired NK cell viability and activity in a dose-dependent manner while modulating tumor cell sensitivity to NK cell-mediated cytotoxicity and the TME, potentially both promoting and impairing NK cell function, depending on dose and tumor heterogeneity, suggesting that combining radiotherapy with strategies to maintain NK cell viability and activity could be beneficial.
References
- Campbell, K.S.; Hasegawa, J. Natural killer cell biology: An update and future directions. J. Allergy Clin. Immunol. 2013, 132, 536–544.
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510.
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The biology of human natural killer-cell subsets. Trends Immunol. 2001, 22, 633–640.
- Zamai, L.; Ahmad, M.; Bennett, I.M.; Azzoni, L.; Alnemri, E.S.; Perussia, B. Natural killer (NK) cell-mediated cytotoxicity: Differential use of TRAIL and Fas ligand by immature and mature primary human NK cells. J. Exp. Med. 1998, 188, 2375–2380.
- Lanier, L.L. Up on the tightrope: Natural killer cell activation and inhibition. Nat. Immunol. 2008, 9, 495–502.
- Veluchamy, J.P.; Kok, N.; van der Vliet, H.J.; Verheul, H.M.W.; de Gruijl, T.D.; Spanholtz, J. The rise of allogeneic Natural killer cells as a platform for cancer immunotherapy: Recent innovations and future developments. Front. Immunol. 2017, 8.
- WHO Key Statistics. Available online: (accessed on 30 October 2020).
- WHO Europe Cancer. Available online: (accessed on 30 October 2020).
- Takeuchi, H.; Maehara, Y.; Tokunaga, E.; Koga, T.; Kakeji, Y.; Sugimachi, K. Prognostic significance of natural killer cell activity in patients with gastric carcinoma: A multivariate analysis. Am. J. Gastroenterol. 2001, 96, 574–578.
- Tartter, P.I.; Steinberg, B.; Barron, D.M.; Martinelli, G. The Prognostic Significance of Natural Killer Cytotoxicity in Patients with Colorectal Cancer. Arch. Surg. 1987, 122, 1264–1268.
- Luna, J.I.; Grossenbacher, S.K.; Murphy, W.J.; Canter, R.J. Natural Killer Cell Immunotherapy Targeting Cancer Stem Cells. Expert Opin. Biol. Ther. 2018, 17, 313–324.
- Battella, S.; Cox, M.C.; Santoni, A.; Palmieri, G. Natural killer (NK) cells and anti-tumor therapeutic mAb: Unexplored interactions. J. Leukoc. Biol. 2016, 99, 87–96.
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199.
- Delaney, G.; Jacob, S.; Featherstone, C.; Barton, M. The role of Radiother in cancer treatment: Estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 2005, 104, 1129–1137.
- Hietanen, T.; Pitkänen, M.; Kapanen, M.; Kellokumpu-Lehtinen, P.L. Post-irradiation viability and cytotoxicity of natural killer cells isolated from human peripheral blood using different methods. Int. J. Radiat. Biol. 2016, 92, 71–79.
- Falcke, S.E.; Rühle, P.F.; Deloch, L.; Fietkau, R.; Frey, B.; Gaipl, U.S. Clinically relevant radiation exposure differentially impacts forms of cell death in human cells of the innate and adaptive immune system. Int. J. Mol. Sci. 2018, 19, 3574.
- Hietanen, T.; Pitkänen, M.; Kapanen, M.; Kellokumpu-Lehtinen, P.L. Effects of single and fractionated irradiation on natural killer cell populations: Radiobiological characteristics of viability and cytotoxicity in vitro. Anticancer Res. 2015, 35, 5193–5200.
- Yang, G.; Kong, Q.; Wang, G.; Jin, H.; Zhou, L.; Yu, D.; Niu, C.; Han, W.; Li, W.; Cui, J. Low-dose ionizing radiation induces direct activation of natural killer cells and provides a novel approach for adoptive cellular immunotherapy. Cancer Biother. Radiopharm. 2014, 29.
- Eric, A.; Juranic, Z.; Tisma, N.; Plesinac, V.; Borojevic, N.; Jovanovic, D.; Milovanovic, Z.; Gavrilovic, D.; Ilic, B. Radiotherapy-induced changes of peripheral blood lymphocyte subpopulations in cervical cancer patients: Relationship to clinical response. J. BUON 2009, 14, 79–83.
- Clave, E.; Socié, G.; Cosset, J.M.; Chaillet, M.P.; Tartour, E.; Girinsky, T.; Carosella, E.; Fridman, H.; Gluckman, E.; Mathiot, C. Multicolor flow cytometry analysis of blood cell subsets in patients given total body irradiation before bone marrow transplantation. Int. J. Radiat. Oncol. Biol. Phys. 1995.
- Louagie, H.; van Eijkeren, M.; Philippe, J.; Thierens, H.; de Ridder, L. Changes in peripheral blood lymphocyte subsets in patients undergoing radiotherapy. Int. J. Radiat. Biol. 1999, 75, 767–771.
- Mozaffari, F.; Lindemalm, C.; Choudhury, A.; Granstam-Björneklett, H.; Helander, I.; Lekander, M.; Mikaelsson, E.; Nilsson, B.; Ojutkangas, M.L.; Österborg, A.; et al. NK-cell and T-cell functions in patients with breast cancer: Effects of surgery and adjuvant chemo- and radiotherapy. Br. J. Cancer 2007, 97, 105–111.
- Nakayama, Y.; Makino, S.; Fukuda, Y.; Ikemoto, T.; Shimizu, A. Varied Effects of Thoracic Irradiation on Peripheral Lymphocyte Subsets in Lung Cancer Patients. Intern. Med. 1995.
- Belka, C.; Ottinger, H.; Kreuzfelder, E.; Weinmann, M.; Lindemann, M.; Lepple-Wienhues, A.; Budach, W.; Grosse-Wilde, H.; Bamberg, M. Impact of localized radiotherapy on blood immune cells counts and function in humans. Radiother. Oncol. 1999.
- Domouchtsidou, A.; Barsegian, V.; Mueller, S.P.; Best, J.; Ertle, J.; Bedreli, S.; Horn, P.A.; Bockisch, A.; Lindemann, M. Impaired lymphocyte function in patients with hepatic malignancies after selective internal radiotherapy. Cancer Immunol. Immunother. 2018.
- Yamaue, H.; Tanimura, H.; Aoki, Y.; Tsunoda, T.; Iwahashi, M.; Tani, M.; Tamai, M.; Noguchi, K.; Kashiwagi, H.; Sasaki, M.; et al. Clinical and immunological evaluation of intraoperative radiation therapy for patients with unresectable pancreatic cancer. J. Surg. Oncol. 1992.
- Blomgren, H.; Baral, E.; Edsmyr, F.; Strender, L.E.; Petrini, B.; Wasserman, J. Natural killer activity in peripheral lymphocyte population following local radiation therapy. Acta Radiol. Oncol. Radiat. Phys. Biol. 1980, 19, 139–143.
- Kim, J.Y.; Son, Y.O.; Park, S.W.; Bae, J.H.; Joo, S.C.; Hyung, H.K.; Chung, B.S.; Kim, S.H.; Kang, C.D. Increase of NKG2D ligands and sensitivity to NK cell-mediated cytotoxicity of tumor cells by heat shock and ionizing radiation. Exp. Mol. Med. 2006, 38, 474–484.
- Fine, J.H.; Chen, P.; Mesci, A.; Allan, D.S.J.; Gasser, S.; Raulet, D.H.; Carlyle, J.R. Chemotherapy-induced genotoxic stress promotes sensitivity to natural killer cell cytotoxicity by enabling missing-self recognition. Cancer Res. 2010, 70, 7102–7113.
- Balaji, G.R.; Aguilar, O.A.; Tanaka, M.; Shingu-Vazquez, M.A.; Fu, Z.; Gully, B.S.; Lanier, L.L.; Carlyle, J.R.; Rossjohn, J.; Berry, R. Recognition of host Clr-b by the inhibitory NKR-P1B receptor provides a basis for missing-self recognition. Nat. Commun. 2018, 9.
- Heo, W.; Lee, Y.S.; Son, C.H.; Yang, K.; Park, Y.S.; Bae, J. Radiation-induced matrix metalloproteinases limit natural killer cell-mediated anticancer immunityin NCI-H23 lung cancer cells. Mol. Med. Rep. 2015, 1800–1806.
- Kim, H.W.; Kim, J.E.; Hwang, M.H.; Jeon, Y.H.; Lee, S.W.; Lee, J.; Zeon, S.K.; Ahn, B.C. Enhancement of Natural Killer Cell Cytotoxicity by Sodium/Iodide Symporter Gene-Mediated Radioiodine Pretreatment in Breast Cancer Cells. PLoS ONE 2013, 8, e70194.
- Garnett, C.T.; Palena, C.; Chakarborty, M.; Tsang, K.Y.; Schlom, J.; Hodge, J.W. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004, 64, 7985–7994.
- Michelin, S.; Gallegos, C.E.; Dubner, D.; Favier, B.; Carosella, E.D. Ionizing radiation modulates the surface expression of human leukocyte antigen-G in a human melanoma cell line. Hum. Immunol. 2009, 70, 1010–1015.
- Urosevic, M.; Kempf, W.; Zagrodnik, B.; Panizzon, R.; Burg, G.; Dummer, R. HLA-G expression in basal cell carcinomas of the skin recurring after radiotherapy. Clin. Exp. Derm. 2005, 422–425.
- Jeong, J.U.; Uong, T.N.T.; Chung, W.K.; Nam, T.K.; Ahn, S.J.; Song, J.Y.; Kim, S.K.; Shin, D.J.; Cho, E.; Kim, K.W.; et al. Effect of irradiation-induced intercellular adhesion molecule-1 expression on natural killer cell-mediated cytotoxicity toward human cancer cells. Cytotherapy 2018, 20, 715–727.
- Ames, E.; Canter, R.J.; Grossenbacher, S.K.; Mac, S.; Smith, R.C.; Monjazeb, A.M.; Chen, M.; Murphy, W.J. Enhanced targeting of stem-like solid tumor cells with radiation and natural killer cells. OncoImmunology 2015, 4, 1–11.
- Shen, M.J.; Xu, L.J.; Yang, L.; Tsai, Y.; Keng, P.C.; Chen, Y.; Lee, S.O.; Chen, Y. Radiation alters PD-L1/NKG2D ligand levels in lung cancer cells and leads to immune escape from NK cell cytotoxicity via IL-6- MEK/Erk signaling pathway. Oncotarget 2017, 8, 80506–80520.
- Multhoff, G.; Botzler, C.; Jennen, L.; Schmidt, J.; Ellwart, J.; Issels, R. Heat shock protein 72 on tumor cells: A recognition structure for natural killer cells. J. Immunol. 1997, 4341–4350.
- Multhoff, G.; Pockley, A.G.; Schmid, T.E.; Schilling, D. The role of heat shock protein 70 (Hsp70) in radiation-induced immunomodulation. Cancer Lett. 2015, 179–184.
- Calini, V.; Urani, C.; Camatini, M. Overexpression of HSP70 is induced by ionizing radiation in C3H 10T1/2 cells and protects from DNA damage. Toxicol. In Vitro 2003, 17, 561–566.
- Gastpar, R.; Gross, C.; Rossbacher, L.; Ellwart, J.; Riegger, J.; Multhoff, G. The Cell Surface-Localized Heat Shock Protein 70 Epitope TKD Induces Migration and Cytolytic Activity Selectively in Human NK Cells. J. Immunol. 2004.
- Multhoff, G.; Mizzen, L.; Winchester, C.C.; Milner, C.M.; Wenk, S.; Eissner, G.; Kampinga, H.H.; Laumbacher, B.; Johnson, J. Heat shock protein 70 (Hsp70) stimulates proliferation and cytolytic activity of natural killer cells. Exp. Hematol. 1999, 27, 1627–1636.
- Stangl, S.; Gross, C.; Pockley, A.G.; Asea, A.A.; Multhoff, G. Influence of Hsp70 and HLA-E on the killing of leukemic blasts by cytokine/Hsp70 peptide-activated human natural killer (NK) cells. Cell Stress Chaperones 2008, 13, 221–230.
- Vaupel, P.; Multhoff, G. Adenosine can thwart anti-tumor immune responses elicited by radiotherapy. Strahlenther. Und Onkol. 2016, 192, 279–287.
- Young, A.; Ngiow, S.F.; Gao, Y.; Patch, A.M.; Barkauskas, D.S.; Messaoudene, M.; Lin, G.; Coudert, J.D.; Stannard, K.A.; Zitvogel, L.; et al. A2AR adenosine signaling suppresses natural killer cell maturation in the tumor microenvironment. Cancer Res. 2018, 78, 1003–1016.
- Yoon, M.S.; Pham, C.T.; Phan, M.T.T.; Shin, D.J.; Jang, Y.Y.; Park, M.H.; Kim, S.K.; Kim, S.; Cho, D. Irradiation of breast cancer cells enhances CXCL16 ligand expression and induces the migration of natural killer cells expressing the CXCR6 receptor. Cytotherapy 2016, 18, 1532–1542.
