Taccalonolides are a new class of microtube-stabilizing agents isolated from plants of the genus Tacca demonstrating effectiveness against drug-resistant tumors in cellular and animal models.
- Taccalonolides
- microtubule stabilizer
- anticancer agent
1. Introduction
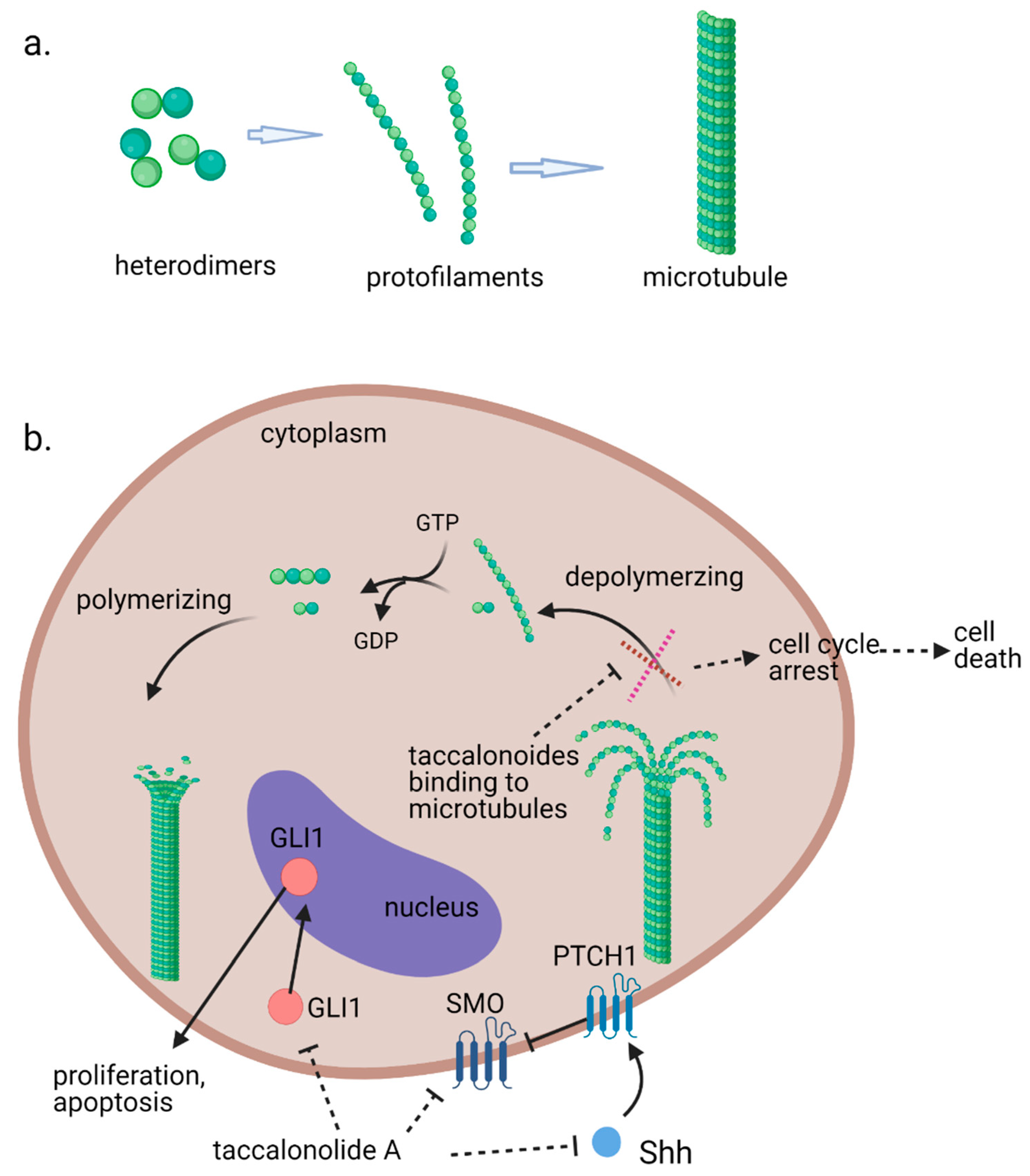
Microtubules, the third principal component of the cytoskeleton, are tube-like long hollow cylindrical filaments found in all eukaryotes and function in a variety of cell movements including transport of organelles, vesicles, or signaling molecules and the separation of chromosomes during mitosis [1][2][1,2]. It has been one of the most successful targets for anticancer drugs including taxanes and vinca alkaloids [3][4][3,4]. Microtubules are composed of a single type of globular protein, called tubulin. The heterodimers of α- and β-tubulin subunits string together to make linear strands named protofilaments in a specific head-to-tail orientation that gives microtubules an innate polarity (Figure 1a) [5][6][7][5,6,7]. In mammalian cells, microtubules generally consist of 13 linear protofilaments arranged in parallel. A characteristic property of microtubules is their ability to undergo continual and rapid cycles of assembly (growth) and disassembly (shrinkage) by adding and subtracting tubulin dimers at both ends of the filament, which is known as dynamic instability [5]. The guanine nucleotide molecule (i.e., GTP) bound to the β-tubulin molecule plays a key role in the dynamic instability. The GTP is hydrolyzed to GDP during or shortly after polymerization. This GTP hydrolysis weakens the binding affinity of tubulin to adjacent molecules and renders the microtubules prone to depolymerization [2][8][2,8]. During mitosis, the microtubule array present in interphase cells disassembles and the free tubulin subunits are reassembled to form the mitotic spindle, which is responsible for the separation of daughter chromosomes [2]. Because of the central role of the rapid reorganization of microtubules in mitosis, microtubules serve as an important and effective target for anticancer drugs (Figure 1b) [9].
Figure 1.
(
a
). Fundamental aspects of microtubule structure. (
b). The dynamic behavior of microtubules and mechanism of action of microtubule-stabilizing agents e.g., taccalonolides. In a recent study, it is observed that mRNA and protein expression levels of sonic hedgehog (Shh), SMO, and GlI1 were decreased in HepG2 and Huh7 cells treated with taccalonolide A. polarity. Aberrant activation of the Shh pathway has been shown in a variety of human cancers. Blockage of Shh pathway induces inhibition of downstream protein smoothened (SMO) at the primary cilium resulting in the decreased nuclear localization of glioma-associated (GLI) transcription factors, preventing the activation of GLI target genes [10].
). The dynamic behavior of microtubules and mechanism of action of microtubule stabilizing agents e.g., taccalonolides. In a recent study, it is observed that mRNA and protein expression levels of sonic hedgehog (Shh), SMO and GlI1 were decreased in HepG2 and Huh7 cells treated with taccalonolide A. polarity. Aberrant activation of the Shh pathway has been shown in a variety of human cancers. Blockage of Shh pathway induces inhibition of downstream protein smoothened (SMO) at the primary cilium resulting in the decreased nuclear localization of glioma-associated (GLI) transcription factors, preventing the activation of GLI target genes [14]. Diagrams were created with BioRender.com (accessed on 12 February 2021).
The microtubule-targeted agents, also referred to as microtubule-binding agents, microtubule-active agents, or antimitotic agents, are often classified into two main groups, the destabilizers and the stabilizers, according to their effects on microtubule polymer mass at high concentrations. The microtubule-stabilizing agents enhance microtubule polymerization at high concentrations and most bind to the same or an overlapping taxane-binding site on β-tubulin such as paclitaxel and docetaxel. Another unique binding site, the peloruside/laulimalide-binding site on α-tubulin was also reported [9][11]. Both microtubule destabilizing and stabilizing agents can suppress microtubule dynamicity, which leads to apoptotic cell death [3][12].
The microtubule-targeted agents, also referred to as microtubule-binding agents, microtubule-active agents, or antimitotic agents, are often classified into two main groups, the destabilizers and the stabilizers, according to their effects on microtubule polymer mass at high concentrations. The microtubule-stabilizing agents enhance microtubule polymerization at high concentrations and most bind to the same or an overlapping taxane-binding site on β-tubulin such as paclitaxel and docetaxel. Another unique binding site, the peloruside/laulimalide-binding site on α-tubulin was also reported [9,10]. Both microtubule destabilizing and stabilizing agents can suppress microtubule dynamicity, which leads to apoptotic cell death [3,11].
In an effort to clarify the bitter principles of the medicinal plant Tacca plantaginea, the structures of isolated taccalonolide A and taccalonolide B were identified in 1987 [13]. The microtubule-disturbing effects of taccalonolides were first discovered in a study conducted by Dr. Mooberry in 2003 following a positive screening result [14]. The taccalonolides have been extensively investigated since then because of their microtubule-stabilizing properties and their effectiveness against drug-resistant cancers in both in vitro and in vivo models.
In an effort to clarify the bitter principles of a medicinal plant Tacca plantaginea, the structures of isolated taccalonolide A and taccalonolide B were identified in 1987 [12]. The microtubule-disturbing effects of taccalonolides were first discovered in a study conducted by Dr. Mooberry in 2003 following a positive screening result [13]. The taccalonolides have been extensively investigated since then because of their microtubule-stabilizing properties and their effectiveness against drug-resistant cancers in both in vitro and in vivo models.
2. In Vitro Antiproliferative Effects and In Vivo Antitumor Efficacy
All isolated taccalonolides exhibit cytotoxicity toward cancer cells (HeLa cells) with a range of potencies from low nanomolar to micromolar levels, except for taccalonolides D and AC [15][16]. An advantage of taccalonolides over the taxanes is their ability to circumvent multiple drug resistance mechanisms. An early study on the evaluation of antiproliferative efficacy of taccalonolides shows that taccalonolides E and A inhibit the proliferation of drug-sensitive cancer cell lines SK-OV-3 and MDA-MB-435, albeit less potent than paclitaxel. However, excitingly, the multidrug-resistant cell lines NCI/ADR, the taxol-resistant cell lines, PTX 10, PTX 22, and the epothilone-resistant cell line, 1A9/A8, are all less resistant to the taccalonolides E and A [14]. In a later study, the susceptibilities to cellular resistance mechanisms including overexpression of P-glycoprotein, MRP7, and the βIII isotype of tubulin are evaluated. Taccalonolides A, E, B, and N are significantly better than paclitaxel at circumventing Pgp-mediated drug resistance both in vitro and in vivo. All four tested taccalonolides also overcome resistance due to the expression of MRP7 and β-tubulin isotypes [17].
All isolated taccalonolides exhibit cytotoxicity toward cancer cells (HeLa cells) with a range of potencies from low nanomolar to micromolar levels, except for taccalonolides D and AC [16,30]. An advantage of taccalonolides over the taxanes is their ability to circumvent multiple drug resistance mechanisms. An early study on the evaluation of antiproliferative efficacy of taccalonolides shows that taccalonolides E and A inhibit the proliferation of drug-sensitive cancer cell lines SK-OV-3 and MDA-MB-435, albeit less potent than paclitaxel. However, excitingly, the multidrug-resistant cell lines NCI/ADR, the taxol-resistant cell lines, PTX 10, PTX 22, and the epothilone-resistant cell line, 1A9/A8, are all less resistant to the taccalonolides E and A [13]. In a later study, the susceptibilities to cellular resistance mechanisms including overexpression of P-glycoprotein, MRP7, and the βIII isotype of tubulin are evaluated. Taccalonolides A, E, B, and N are significantly better than paclitaxel at circumventing Pgp-mediated drug resistance both in vitro and in vivo. All four tested taccalonolides also overcome resistance due to the expression of MRP7 and β-tubulin isotypes [33].
A recent study suggests that inhibition of the activation of the sonic hedgehog (Shh) pathway also contributes to the antiproliferative effects of taccalonolide A against Hepatocellular carcinoma (HCC) cells [18]. Taccalonolide A represses cell viability and accelerates apoptosis of HepG2 and Huh7 cells. It is observed that mRNA and protein expression levels of Shh, SMO and GlI1 were decreased in taccalonolide A treated HCC cells (Figure 1b).
A recent study suggests that inhibition of the activation of the sonic hedgehog (Shh) pathway also contributes to the antiproliferative effects of taccalonolide A against Hepatocellular carcinoma (HCC) cells [32]. Taccalonolide A represses cell viability and accelerates apoptosis of HepG2 and Huh7 cells. It is observed that mRNA and protein expression levels of Shh, SMO and GlI1 were decreased in taccalonolide A treated HCC cells (Figure 1b).
It is found that taccalonolides A and E are substantially more potent in vivo than would be expected from their in vitro potency [17]. Additionally, the in vivo evaluation suggests that taccalonolides have narrow therapeutical windows. For instance, a dose of 56 mg/kg taccalonolide A providing the longest tumor growth delay and the highest gross log cell kill is above the maximum tolerated dose. The same effects are observed with more potent taccalonolide AF [16][19]. Taccalonolide AF is found to have potent antitumor effects with inhibition of tumor growth almost identical to that of 10 mg/kg paclitaxel when a 2.0 mg/kg dose is administered. A slightly higher dose of 2.5 mg/kg with a cumulative dose of 5.0 mg/kg causes significant weight loss and toxicity. The lack of antitumor efficacy at doses lower than the LD
It is found that taccalonolides A and E are substantially more potent in vivo than would be expected from their in vitro potency [33]. Additionally, the in vivo evaluation suggests that taccalonolides have narrow therapeutical windows. For instance, a dose of 56 mg/kg taccalonolide A providing the longest tumor growth delay and the highest gross log cell kill is above the maximum tolerated dose. The same effects are observed with more potent taccalonolide AF [30,40]. Taccalonolide AF is found to have potent antitumor effects with inhibition of tumor growth almost identical to that of 10 mg/kg paclitaxel when a 2.0 mg/kg dose is administered. A slightly higher dose of 2.5 mg/kg with a cumulative dose of 5.0 mg/kg causes significant weight loss and toxicity. The lack of antitumor efficacy at doses lower than the LD
80 leads taccalonolide AJ absent of any therapeutic window in the MDA-MB-231 breast cancer xenograft model [19]. However, AJ has excellent and persistent antitumor efficacy when it is intratumorally injected into the tumor. Its short half-life (t
leads taccalonolide AJ absent of any therapeutic window in the MDA-MB-231 breast cancer xenograft model [40]. However, AJ has excellent and persistent antitumor efficacy when it is intratumorally injected into the tumor. Its short half-life (t
1/2) of 8.1 min limits its delivery to the tumor and precluded it from achieving antitumor efficacy at systemically tolerable doses [20]. A cyclodextrin inclusion complex of AJ has been developed to improve the therapeutic window and reduced toxicity [21]. It is also observed that the mitotic arrest and the antiproliferative effects of taccalonolides are more persistent and less reversible than the other microtubule disrupting agents evaluated, paclitaxel and laulimalide [22]. Similarly, two potent semi-synthetic taccalonolides, bearing isovalerate modifications at C-7 or C-15, demonstrate highly persistent in vivo antitumor activity in a drug-resistant xenograft model [23].
) of 8.1 min limits its delivery to the tumor and precluded it from achieving antitumor efficacy at systemically tolerable doses [50]. A cyclodextrin inclusion complex of AJ has been developed to improve the therapeutic window and reduced toxicity [51]. It is also observed that the mitotic arrest and the antiproliferative effects of taccalonolides are more persistent and less reversible than the other microtubule disrupting agents evaluated, paclitaxel and laulimalide [28]. Similarly, two potent semi-synthetic taccalonolides, bearing isovalerate modifications at C-7 or C-15, demonstrate highly persistent in vivo antitumor activity in a drug-resistant xenograft model [39].
3. Conclusions
Taccalonolides are the first class of covalently bound microtubule stabilizers displaying potent anticancer properties and, undoubtedly, are a group of attractive therapeutic candidates for cancer treatment [24][15]. Taccalonolides have shown excellent efficacy in drug-resistant models both in vitro and in vivo. Long-lasting therapeutic responses and high cellular persistence of taccalonolides are observed in murine xenograft models and clonogenic assay. One of the major challenges to further develop taccalonolides for clinical use is that taccalonolides has a narrow therapeutic window when administered systemically. The potent fluorescein-labeled taccalonolides synthesized in this study suggest that targeted drug delivery, such as by attaching an antibody to taccalonolides, might be an efficacious strategy to reduce the optimal drug dose required for a significant antitumor effect and manage systemic toxicities [25][26][18,46]. In the last several years, understanding of SARs and the mechanism of action of taccalonolides has advanced significantly, which will facilitate the discovery and designing of new chemical entities with potent and selective anti-cancer activities. In summary, as a new generation of microtubule stabilizers, taccalonolides have shown promising potential for the treatment of cancers with exciting advantages over current anticancer agents.