Even though major depressive disorder (MDD) and post-traumatic stress disorder (PTSD) are among the most prevalent and incapacitating mental illnesses in the world, their diagnosis still relies solely on the characterization of subjective symptoms (many of which are shared by multiple disorders) self-reported by patients and biomarkers to facilitate diagnosis and treatment are still an unmet necessity.
- allopregnanolone
- BDNF
- PTSD
- MDD
- Biomarkers
1. Introduction
Major depressive disorder (MDD) and post-traumatic stress disorder (PTSD) are among the most prevalent, debilitating, and incapacitating mental illnesses that pose a significant disease burden and loss of adjusted life years [1]. Both disorders are believed to emerge as a result of a maladaptive response to stressful events in individuals who fail to develop resilience. MDD and PTSD have similar etiology, manifest overlapping symptoms, and MDD is often seen as a worsening condition in PTSD, which can be accompanied by suicide. The shared symptomatology is relevant because the clinical diagnosis of MDD and PTSD is still based on subjective rather than objective measures that are entirely based on symptom evaluation, following criteria defined by the Diagnostic and Statistical Manual of Mental Disorders (DSM)-V. Even though the DSM-V offers a structured set of symptoms for each disorder, the over reliance on subjective self-reports of symptoms contributes to diagnoses that are misleading at times, mainly in the first medical visits, and in cases characterized by a mild to moderate depression and distress [2]. For this reason, the addition of an array of objective measures of neurobiological parameters would warrant a significant boost in the diagnosis accuracy of psychiatric disorders, including PTSD and MDD. Great progress has been achieved in the study of neurochemical deficits that underlie the manifestation of psychiatric disorders, and several novel biomarker candidates to help in the diagnosis of PTSD and MDD have been proposed.
2. Biomarkers for Psychiatric Disorders: An Unmet Need
The term “biomarker” refers to a broad category of measurable indicators of the existence or the severity of a disease/disorder, being objective indications of medical state observed from the patient, which can be measured in an accurate and reproducible manner. Thus, biomarkers objectively indicate the pathological state of a subject that correlate to assessed medical symptoms, which are limited to indications perceived by the patient on their mental health disorders. The Biomarkers Definitions Working Group provided an international definition of biomarker for the field of pharmacological clinical trials: “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention” [3].
The development of reliable biomarkers for psychiatric disorders depends on a large understanding of the pathological processes that underlie the specific neuropathology under examination. This can be particularly challenging for psychiatric disorders because of their broad symptomatic characterization and the lack of a sufficiently detailed understanding of the functional abnormalities, either neural or somatic, associated with psychiatric disorders. Nevertheless, significant progress in this field has been made in the last decades, and the development of reliable biomarkers for MDD and PTSD is becoming a realistic possibility.
There are several kinds of biomarkers that have been proposed for the diagnosis and prognosis of MDD and PTSD, including genetic and epigenetic markers, proteins, and neurohormones [4]. Each of these parameters attempts to reflect part of a theoretical axis of biological alterations that gives origin to symptoms or to the targeted disorders. The origin of such alterations is believed to be largely dependent on the exposure to stressful events, and a complex combination of factors that include the nature of the stressor and the individual’s construct may result in one disorder instead of another. Exposure to stress in a chronic, repeated fashion is believed to be an important risk factor for the development of MDD, while exposure to acute, yet intense, traumatic events, often in individuals that experience chronic stress conditions, may precipitate the development of PTSD. Given that protracted and acute stress play an important role in the etiology of these disorders, the body’s physiological regulation of the stress response becomes an important point of study to contextualize biochemical alterations in MDD and PTSD.
3. Neurosteroids
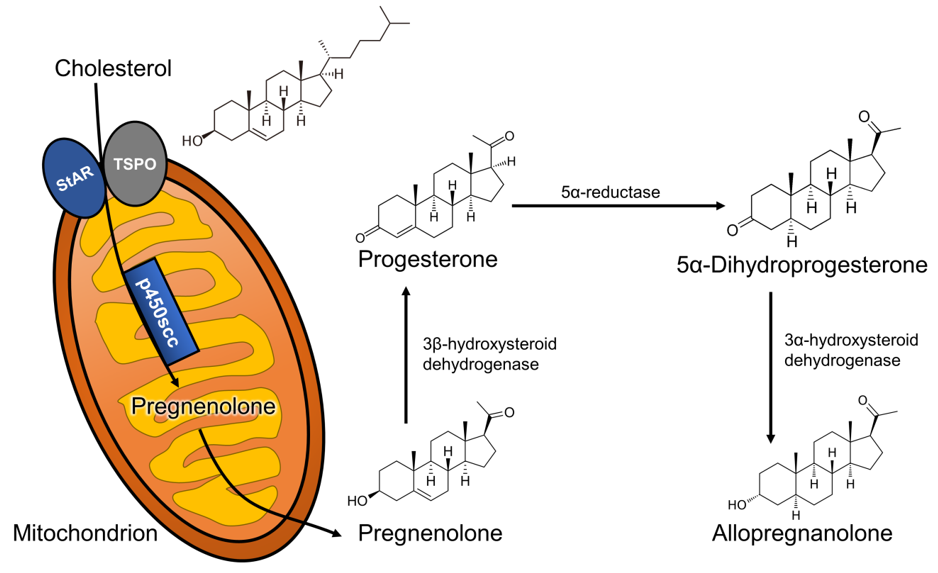
Neurosteroids are endogenous steroids synthesized in the central and peripheral nervous system from cholesterol [5] and belong to the broader category of neuroactive steroids, which also include peripherally or artificially synthesized steroids with activity in the brain [6]. The synthesis of neurosteroids, or neurosteroidogenesis, follows a few distinct steps: (1) cholesterol is trafficked to the outer mitochondrial membrane by action of the steroidogenic acute regulatory (StAR) protein; (2) cholesterol is then transported into the inner mitochondrial membrane by the 18 kDa translocator protein (TSPO); (3) its side-chain is cleaved by the CYP11A1 enzyme, which produces the neurosteroid precursor, pregnenolone. Pregnenolone can then be converted to progesterone, which originates a wide array of steroid-derived molecules that include sex and stress hormones, as well as endogenous neuroactive steroids [7]. In this review, we will focus on the neurosteroid pathway (represented in Figure 1) that begins with the reduction of progesterone by the action of the 5α-reductase type I (5α-RI) enzyme, into 5α-dihydroprogesterone, which is subsequently converted by the 3α-hydroxysteroid dehydrogenase (3α-HSD) type III enzyme to 3α,5α-tetrahydroprogesterone (commonly known as allopregnanolone).
Neurosteroids rapidly modulate neuronal excitability due to their affinity to ligand-gated ion channels and other receptors expressed in the synaptosomal membranes of brain cells and neurons. Arguably, the most important neuronal action exerted by neurosteroids is related to the modulation of the inhibitory activity mediated by the neurotransmitter GABA through the allosteric potentiation of the GABAA receptor [8–10]. Allopregnanolone and its stereoisomer, pregnanolone, are among the most potent neurosteroids in positively and allosterically modulating GABAA receptors. These GABAergic steroids are found diminished in the cerebrospinal fluid (CSF) [11], serum [12], and plasma [13] of depressed individuals. The expression of the 5α-RI enzyme, which is essential for allopregnanolone synthesis, is reduced in the postmortem prefrontal cortex (Brodman area 9) of depressed individuals [14]. Exogenous administration of allopregnanolone and other GABAergic synthetic neurosteroid analogs resulted in antidepressant and anxiolytic effects, which is believed to be partly due to the role neurosteroids play in the modulation of GABAergic inhibition, thereby also reducing depressive symptoms [15]. The reduced allopregnanolone in the CSF and plasma of depressed individuals [11–13] can be increased after antidepressant treatment with selective serotonin reuptake inhibitors (SSRIs), including fluoxetine and fluvoxamine [12].

Figure 1. Allopregnanolone biosynthetic pathway starting from cholesterol metabolism. Abbreviations: StAR: steroidogenic acute regulatory protein; TSPO: 18 kDa translocator protein; scc: side-chain cleavage.
Allopregnanolone biosynthetic pathway starting from cholesterol metabolism. Abbreviations: StAR: steroidogenic acute regulatory protein; TSPO: 18 kDa translocator protein; scc: side-chain cleavage.
Affective disorders, including MDD and PTSD, show a strong sex-bias being more prevalent in women than in men, which suggests that sex steroids may play a role. Both progesterone and its metabolite, allopregnanolone, have been the focus of several investigations looking at sex-specific roles of neurosteroids in depressive disorders. Lower serum allopregnanolone levels have also been observed in women suffering from postpartum depression (PPD) [16]. Significant fluctuations in progesterone and allopregnanolone levels during pregnancy, which rise considerably before abruptly decreasing following delivery, may be responsible for the development of PPD pathophysiology [17,18]. From a predictive perspective, the second trimester of pregnancy seems to play an important factor and should be considered in the development of PPD symptoms during the last days of pregnancy and in the post-partum period [19,20].
Neurosteroids rapidly modulate neuronal excitability due to their affinity to ligand-gated ion channels and other receptors expressed in the synaptosomal membranes of brain cells and neurons. Arguably, the most important neuronal action exerted by neurosteroids is related to the modulation of the inhibitory activity mediated by the neurotransmitter GABA through the allosteric potentiation of the GABAA receptor [8][9][10]. Allopregnanolone and its stereoisomer, pregnanolone, are among the most potent neurosteroids in positively and allosterically modulating GABAA receptors. These GABAergic steroids are found diminished in the cerebrospinal fluid (CSF) [11], serum [12], and plasma [13] of depressed individuals. The expression of the 5α-RI enzyme, which is essential for allopregnanolone synthesis, is reduced in the postmortem prefrontal cortex (Brodman area 9) of depressed individuals [14]. Exogenous administration of allopregnanolone and other GABAergic synthetic neurosteroid analogs resulted in antidepressant and anxiolytic effects, which is believed to be partly due to the role neurosteroids play in the modulation of GABAergic inhibition, thereby also reducing depressive symptoms [15]. The reduced allopregnanolone in the CSF and plasma of depressed individuals [11][12][13] can be increased after antidepressant treatment with selective serotonin reuptake inhibitors (SSRIs), including fluoxetine and fluvoxamine [12].
In female patients with PTSD, CSF, serum, and plasma allopregnanolone levels are also lower than in healthy age-matched controls, which has been associated with enzymatic dysfunction at the levels of 3α-HSD [21,22]. Additionally, women with PTSD show a lower capacity for allopregnanolone synthesis from its precursor, which was confirmed in serum samples [23,24]. Similarly, in males with PTSD, the concentrations of allopregnanolone in the CSF were found lower than in healthy controls [25]. Ratios with allopregnanolone precursors supported a 5α-RI expression/function abnormality. In these patients, the allopregnanolone level decrease was inversely correlated with increased PTSD and depression symptoms. These findings align with previous results that observed a 5α-RI expression downregulation in the human post-mortem brain (Brodmann area 9) of male depressed subjects. Intriguingly, the enzymatic deficits in the allopregnanolone pathway may underlay a biosignature relevant for psychopathology related to dysfunction in reproductive steroid biosynthesis. Allopregnanolone levels were also found to be reduced in the medial orbital frontal cortex of individuals with PTSD in comparison to controls, using postmortem brain tissue samples [26]. A recent study showed that PTSD symptoms were inversely correlated with combined CSF levels of allopregnanolone and pregnanolone, which was not observed in matched trauma-exposed controls [25]. These results are believed to correlate with decreased brain allopregnanolone levels, which are observed in the limbic system areas that are relevant to affect regulation in several animal models of depression and of PTSD (for an in-depth review on this topic, see [27–29]). Even though MDD and PTSD share some neurobiological aspects, it is timely to investigate if allopregnanolone downregulation follows a distinct trajectory between the two disorders. Further, it is conceivable that allopregnanolone and pregnanolone levels are compared to the levels of their precursors, calculating ratios that estimate their synthesis rate and also investigating the function and/or expression of the biosynthetic enzymes.
Affective disorders, including MDD and PTSD, show a strong sex-bias being more prevalent in women than in men, which suggests that sex steroids may play a role. Both progesterone and its metabolite, allopregnanolone, have been the focus of several investigations looking at sex-specific roles of neurosteroids in depressive disorders. Lower serum allopregnanolone levels have also been observed in women suffering from postpartum depression (PPD) [16]. Significant fluctuations in progesterone and allopregnanolone levels during pregnancy, which rise considerably before abruptly decreasing following delivery, may be responsible for the development of PPD pathophysiology [17][18]. From a predictive perspective, the second trimester of pregnancy seems to play an important factor and should be considered in the development of PPD symptoms during the last days of pregnancy and in the post-partum period [19][20].
In humans, CSF measurements of neurosteroids are expected to reflect brain levels. However, a study conducted in male and female rats showed a poor correlation of CSF allopregnanolone levels with its content in relevant brain areas, such as the hippocampus and cerebral cortex [30]. Plasma levels were surprisingly predictive of cerebral cortex levels [30], encouraging the extrapolation of peripheral neurosteroid findings to the CNS. Another study in humans found a significantly more robust correlation between CSF and serum free allopregnanolone levels [31]. In human studies in males, however, correlation of steroid levels in the CSF and plasma were either weak or very weak [32]. On the other hand, regardless of the precise correlation within subjects, plasma allopregnanolone levels have been shown to reflect the directional changes predicted in the brain. In addition to plasma, the non-invasive sampling of saliva could offer an alternative to blood draws for the quantification of neurosteroids in that it would reduce the stress associated with blood collection that could affect circulating neuroactive steroid levels, and thereby confounding interpretation of the experimental results. To our knowledge, there have not yet been studies comparing allopregnanolone levels in saliva with blood or CSF levels, but the reliable use of cortisol measurement in saliva [33], suggests that this may be an appropriate direction to focus in future research. Similarly, salivary progesterone levels successfully reflect plasma levels, as confirmed also in studies conducted during pregnancy [34].
In female patients with PTSD, CSF, serum, and plasma allopregnanolone levels are also lower than in healthy age-matched controls, which has been associated with enzymatic dysfunction at the levels of 3α-HSD [21][22]. Additionally, women with PTSD show a lower capacity for allopregnanolone synthesis from its precursor, which was confirmed in serum samples [23][24]. Similarly, in males with PTSD, the concentrations of allopregnanolone in the CSF were found lower than in healthy controls [25]. Ratios with allopregnanolone precursors supported a 5α-RI expression/function abnormality. In these patients, the allopregnanolone level decrease was inversely correlated with increased PTSD and depression symptoms. These findings align with previous results that observed a 5α-RI expression downregulation in the human post-mortem brain (Brodmann area 9) of male depressed subjects. Intriguingly, the enzymatic deficits in the allopregnanolone pathway may underlay a biosignature relevant for psychopathology related to dysfunction in reproductive steroid biosynthesis. Allopregnanolone levels were also found to be reduced in the medial orbital frontal cortex of individuals with PTSD in comparison to controls, using postmortem brain tissue samples [26]. A recent study showed that PTSD symptoms were inversely correlated with combined CSF levels of allopregnanolone and pregnanolone, which was not observed in matched trauma-exposed controls [25]. These results are believed to correlate with decreased brain allopregnanolone levels, which are observed in the limbic system areas that are relevant to affect regulation in several animal models of depression and of PTSD (for an in-depth review on this topic, see [27][28][29]). Even though MDD and PTSD share some neurobiological aspects, it is timely to investigate if allopregnanolone downregulation follows a distinct trajectory between the two disorders. Further, it is conceivable that allopregnanolone and pregnanolone levels are compared to the levels of their precursors, calculating ratios that estimate their synthesis rate and also investigating the function and/or expression of the biosynthetic enzymes.
Altogether these summaries suggest that more studies should be conducted to establish clear correlation among central neurosteroid biosynthesis changes with those occurring in the periphery, including blood and saliva. These investigations will be crucial in assessing a putative biomarker role of neurosteroids in psychiatric disorders.
In humans, CSF measurements of neurosteroids are expected to reflect brain levels. However, a study conducted in male and female rats showed a poor correlation of CSF allopregnanolone levels with its content in relevant brain areas, such as the hippocampus and cerebral cortex [30]. Plasma levels were surprisingly predictive of cerebral cortex levels [30], encouraging the extrapolation of peripheral neurosteroid findings to the CNS. Another study in humans found a significantly more robust correlation between CSF and serum free allopregnanolone levels [31]. In human studies in males, however, correlation of steroid levels in the CSF and plasma were either weak or very weak [32]. On the other hand, regardless of the precise correlation within subjects, plasma allopregnanolone levels have been shown to reflect the directional changes predicted in the brain. In addition to plasma, the non-invasive sampling of saliva could offer an alternative to blood draws for the quantification of neurosteroids in that it would reduce the stress associated with blood collection that could affect circulating neuroactive steroid levels, and thereby confounding interpretation of the experimental results. To our knowledge, there have not yet been studies comparing allopregnanolone levels in saliva with blood or CSF levels, but the reliable use of cortisol measurement in saliva [33], suggests that this may be an appropriate direction to focus in future research. Similarly, salivary progesterone levels successfully reflect plasma levels, as confirmed also in studies conducted during pregnancy [34].
References
- Rehm, J.; Shield, K.D. Global Burden of Disease and the Impact of Mental and Addictive Disorders. Psychiatry Rep. 2019, 21, 10, doi:10.1007/s11920-019-0997-0.
- Aspesi, D.; Pinna, G. Could a Blood Test for PTSD and Depression Be on the Horizon? Expert Rev. Proteom. 2018, 15, 983–1006, doi:10.1080/14789450.2018.1544894.
- Biomarkers Definitions Working Group; Atkinson, A.J., Jr.; Colburn, W.A.; DeGruttola, V.G.; DeMets, D.L.; Downing, G.J.; Hoth, D.F.; Oates, J.A.; Peck, C.C.; Schooley, R.T.; et al. Biomarkers and Surrogate Endpoints: Preferred Definitions and Conceptual Framework. Pharmacol. Ther. 2001, 69, 89–95, doi:10.1067/mcp.2001.113989.
- Huang, T.-L.; Lin, C.-C. Chapter Seven—Advances in Biomarkers of Major Depressive Disorder. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 68, pp. 177–204.
- Baulieu, E.E.; Robel, P.; Schumacher, M. Neurosteroids: Beginning of the story. In International Review of Neurobiology; Elsevier: Amsterdam, The Netherlands, 2001; Volume 46, pp. 1–32, ISBN 978-0-12-366846-2.
- Paul, S.M.; Purdy, R.H. Neuroactive Steroids. J. 1992, 6, 2311–2322, doi:10.1096/fasebj.6.6.1347506.
- Mellon, S.H.; Griffin, L.D.; Compagnone, N.A. Biosynthesis and Action of Neurosteroids. Brain Res. Rev. 2001, 37, 3–12, doi:10.1016/S0165-0173(01)00109-6.
- Paul, S.M.; Pinna, G.; Guidotti, A. Allopregnanolone: From Molecular Pathophysiology to Therapeutics. A Historical Perspective. Stress 2020, 12, 100215, doi:10.1016/j.ynstr.2020.100215.
- Puia, G.; Vicini, S.; Seeburg, P.H.; Costa, E. Influence of Recombinant Gamma-Aminobutyric Acid-A Receptor Subunit Composition on the Action of Allosteric Modulators of Gamma-Aminobutyric Acid-Gated Cl- Currents. Pharmacol. 1991, 39, 691–696.
- Reddy, D.S. Neurosteroids. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2010; Volume 186, pp. 113–137, ISBN 978-0-444-53630-3.
- Uzunova, V.; Sheline, Y.; Davis, J.M.; Rasmusson, A.; Uzunov, D.P.; Costa, E.; Guidotti, A. Increase in the Cerebrospinal Fluid Content of Neurosteroids in Patients with Unipolar Major Depression Who Are Receiving Fluoxetine or Fluvoxamine. Natl. Acad. Sci. USA 1998, 95, 3239–3244, doi:10.1073/pnas.95.6.3239.
- Romeo, E.; Ströhle, A.; Spalletta, G.; di Michele, F.; Hermann, B.; Holsboer, F.; Pasini, A.; Rupprecht, R. Effects of Antidepressant Treatment on Neuroactive Steroids in Major Depression. AJP 1998, 155, 910–913, doi:10.1176/ajp.155.7.910.
- Schüle, C.; Romeo, E.; Uzunov, D.P.; Eser, D.; di Michele, F.; Baghai, T.C.; Pasini, A.; Schwarz, M.; Kempter, H.; Rupprecht, R. Influence of Mirtazapine on Plasma Concentrations of Neuroactive Steroids in Major Depression and on 3α-Hydroxysteroid Dehydrogenase Activity. Psychiatry 2006, 11, 261–272, doi:10.1038/sj.mp.4001782.
- Agis-Balboa, R.C.; Guidotti, A.; Pinna, G. 5α-Reductase Type I Expression Is Downregulated in the Prefrontal Cortex/Brodmann’s Area 9 (BA9) of Depressed Patients. Psychopharmacology 2014, 231, 3569–3580, doi:10.1007/s00213-014-3567-5.
- Maguire, J. Neuroactive Steroids and GABAergic Involvement in the Neuroendocrine Dysfunction Associated with Major Depressive Disorder and Postpartum Depression. Cell. Neurosci. 2019, 13, 83, doi:10.3389/fncel.2019.00083.
- Nappi, R.E.; Petraglia, F.; Luisi, S.; Polatti, F.; Farina, C.; Genazzani, A.R. Serum Allopregnanolone in Women with Postpartum “Blues”. Gynecol. 2001, 97, 77–80, doi:10.1016/S0029-7844(00)01112-1.
- Zorumski, C.F.; Paul, S.M.; Izumi, Y.; Covey, D.F.; Mennerick, S. Neurosteroids, Stress and Depression: Potential Therapeutic Opportunities. Biobehav. Rev. 2013, 37, 109–122, doi:10.1016/j.neubiorev.2012.10.005.
- Zorumski, C.F.; Paul, S.M.; Covey, D.F.; Mennerick, S. Neurosteroids as Novel Antidepressants and Anxiolytics: GABA-A Receptors and Beyond. Stress 2019, 11, 100196, doi:10.1016/j.ynstr.2019.100196.
- McEvoy, K.; Payne, J.L.; Osborne, L.M. Neuroactive Steroids and Perinatal Depression: A Review of Recent Literature. Psychiatry Rep. 2018, 20, 78, doi:10.1007/s11920-018-0937-4.
- Osborne, L.M.; Gispen, F.; Sanyal, A.; Yenokyan, G.; Meilman, S.; Payne, J.L. Lower Allopregnanolone during Pregnancy Predicts Postpartum Depression: An Exploratory Study. Psychoneuroendocrinology 2017, 79, 116–121, doi:10.1016/j.psyneuen.2017.02.012.
- Rasmusson, A.M.; Pinna, G.; Paliwal, P.; Weisman, D.; Gottschalk, C.; Charney, D.; Krystal, J.; Guidotti, A. Decreased Cerebrospinal Fluid Allopregnanolone Levels in Women with Posttraumatic Stress Disorder. Psychiatry 2006, 60, 704–713, doi:10.1016/j.biopsych.2006.03.026.
- Pinna, G. Allopregnanolone, the Neuromodulator Turned Therapeutic Agent: Thank You, Next? Endocrinol. 2020, 11, 236, doi:10.3389/fendo.2020.00236.
- Pineles, S.L.; Nillni, Y.I.; Pinna, G.; Irvine, J.; Webb, A.; Arditte Hall, K.A.; Hauger, R.; Miller, M.W.; Resick, P.A.; Orr, S.P.; et al. PTSD in Women Is Associated with a Block in Conversion of Progesterone to the GABAergic Neurosteroids Allopregnanolone and Pregnanolone Measured in Plasma. Psychoneuroendocrinology 2018, 93, 133–141, doi:10.1016/j.psyneuen.2018.04.024.
- Pineles, S.L.; Nillni, Y.I.; Pinna, G.; Webb, A.; Arditte Hall, K.A.; Fonda, J.R.; Irvine, J.; King, M.W.; Hauger, R.L.; Resick, P.A.; et al. Associations between PTSD-Related Extinction Retention Deficits in Women and Plasma Steroids That Modulate Brain GABAA and NMDA Receptor Activity. Stress 2020, 13, 100225, doi:10.1016/j.ynstr.2020.100225.
- Rasmusson, A.M.; King, M.W.; Valovski, I.; Gregor, K.; Scioli-Salter, E.; Pineles, S.L.; Hamouda, M.; Nillni, Y.I.; Anderson, G.M.; Pinna, G. Relationships between Cerebrospinal Fluid GABAergic Neurosteroid Levels and Symptom Severity in Men with PTSD. Psychoneuroendocrinology 2019, 102, 95–104, doi:10.1016/j.psyneuen.2018.11.027.
- Cruz, D.A.; Glantz, L.A.; McGaughey, K.D.; Parke, G.; Shampine, L.J.; Kilts, J.D.; Naylor, J.C.; Marx, C.E.; Williamson, D.E. Neurosteroid Levels in the Orbital Frontal Cortex of Subjects with PTSD and Controls: A Preliminary Report. Chronic Stress 2019, 3, 247054701983857, doi:10.1177/2470547019838570.
- Almeida, F.B.; Nin, M.S.; Barros, H.M.T. The Role of Allopregnanolone in Depressive-like Behaviors: Focus on Neurotrophic Proteins. Stress 2020, 12, 100218, doi:10.1016/j.ynstr.2020.100218.
- Locci, A.; Pinna, G. Neurosteroid Biosynthesis Down-Regulation and Changes in GABA A Receptor Subunit Composition: A Biomarker Axis in Stress-Induced Cognitive and Emotional Impairment: Neurosteroids and GABA: Biomarkers for Emotions. J. Pharmacol. 2017, 174, 3226–3241, doi:10.1111/bph.13843.
- Pinna, G. Animal Models of PTSD: The Socially Isolated Mouse and the Biomarker Role of Allopregnanolone. Behav. Neurosci. 2019, 13, 114, doi:10.3389/fnbeh.2019.00114.
- Caruso, D.; Pesaresi, M.; Abbiati, F.; Calabrese, D.; Giatti, S.; Garcia-Segura, L.M.; Melcangi, R.C. Comparison of Plasma and Cerebrospinal Fluid Levels of Neuroactive Steroids with Their Brain, Spinal Cord and Peripheral Nerve Levels in Male and Female Rats. Psychoneuroendocrinology 2013, 38, 2278–2290, doi:10.1016/j.psyneuen.2013.04.016.
- Kancheva, R.; Hill, M.; Novák, Z.; Chrastina, J.; Velíková, M.; Kancheva, L.; Říha, I.; Stárka, L. Peripheral Neuroactive Steroids May Be as Good as the Steroids in the Cerebrospinal Fluid for the Diagnostics of CNS Disturbances. Steroid Biochem. Mol. Biol. 2010, 119, 35–44, doi:10.1016/j.jsbmb.2009.12.006.
- Martin, J.; Plank, E.; Jungwirth, B.; Hapfelmeier, A.; Podtschaske, A.; Kagerbauer, S.M. Weak Correlations between Serum and Cerebrospinal Fluid Levels of Estradiol, Progesterone and Testosterone in Males. BMC Neurosci. 2019, 20, 53, doi:10.1186/s12868-019-0535-3.
- Vining, R.F.; McGinley, R.A. The Measurement of Hormones in Saliva: Possibilities and Pitfalls. Steroid Biochem. 1987, 27, 81–94, doi:10.1016/0022-4731(87)90297-4.
- Meulenberg, P.M.; Hofman, J.A. Salivary Progesterone Excellently Reflects Free and Total Progesterone in Plasma during Pregnancy. Chem. 1989, 35, 168–172, doi:10.1093/clinchem/35.1.168.
Altogether these summaries suggest that more studies should be conducted to establish clear correlation among central neurosteroid biosynthesis changes with those occurring in the periphery, including blood and saliva. These investigations will be crucial in assessing a putative biomarker role of neurosteroids in psychiatric disorders.
References
- Rehm, J.; Shield, K.D. Global Burden of Disease and the Impact of Mental and Addictive Disorders. Curr. Psychiatry Rep. 2019, 21, 10.
- Aspesi, D.; Pinna, G. Could a Blood Test for PTSD and Depression Be on the Horizon? Expert Rev. Proteom. 2018, 15, 983–1006.
- Biomarkers Definitions Working Group; Atkinson, A.J., Jr.; Colburn, W.A.; DeGruttola, V.G.; DeMets, D.L.; Downing, G.J.; Hoth, D.F.; Oates, J.A.; Peck, C.C.; Schooley, R.T.; et al. Biomarkers and Surrogate Endpoints: Preferred Definitions and Conceptual Framework. Clin. Pharmacol. Ther. 2001, 69, 89–95.
- Huang, T.-L.; Lin, C.-C. Chapter Seven—Advances in Biomarkers of Major Depressive Disorder. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 68, pp. 177–204.
- Baulieu, E.E.; Robel, P.; Schumacher, M. Neurosteroids: Beginning of the story. In International Review of Neurobiology; Elsevier: Amsterdam, The Netherlands, 2001; Volume 46, pp. 1–32. ISBN 978-0-12-366846-2.
- Paul, S.M.; Purdy, R.H. Neuroactive Steroids. FASEB J. 1992, 6, 2311–2322.
- Mellon, S.H.; Griffin, L.D.; Compagnone, N.A. Biosynthesis and Action of Neurosteroids. Brain Res. Rev. 2001, 37, 3–12.
- Paul, S.M.; Pinna, G.; Guidotti, A. Allopregnanolone: From Molecular Pathophysiology to Therapeutics. A Historical Perspective. Neurobiol. Stress 2020, 12, 100215.
- Puia, G.; Vicini, S.; Seeburg, P.H.; Costa, E. Influence of Recombinant Gamma-Aminobutyric Acid-A Receptor Subunit Composition on the Action of Allosteric Modulators of Gamma-Aminobutyric Acid-Gated Cl- Currents. Mol. Pharmacol. 1991, 39, 691–696.
- Reddy, D.S. Neurosteroids. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2010; Volume 186, pp. 113–137. ISBN 978-0-444-53630-3.
- Uzunova, V.; Sheline, Y.; Davis, J.M.; Rasmusson, A.; Uzunov, D.P.; Costa, E.; Guidotti, A. Increase in the Cerebrospinal Fluid Content of Neurosteroids in Patients with Unipolar Major Depression Who Are Receiving Fluoxetine or Fluvoxamine. Proc. Natl. Acad. Sci. USA 1998, 95, 3239–3244.
- Romeo, E.; Ströhle, A.; Spalletta, G.; di Michele, F.; Hermann, B.; Holsboer, F.; Pasini, A.; Rupprecht, R. Effects of Antidepressant Treatment on Neuroactive Steroids in Major Depression. Am. J. Psychiatry 1998, 155, 910–913.
- Schüle, C.; Romeo, E.; Uzunov, D.P.; Eser, D.; di Michele, F.; Baghai, T.C.; Pasini, A.; Schwarz, M.; Kempter, H.; Rupprecht, R. Influence of Mirtazapine on Plasma Concentrations of Neuroactive Steroids in Major Depression and on 3α-Hydroxysteroid Dehydrogenase Activity. Mol. Psychiatry 2006, 11, 261–272.
- Agis-Balboa, R.C.; Guidotti, A.; Pinna, G. 5α-Reductase Type I Expression Is Downregulated in the Prefrontal Cortex/Brodmann’s Area 9 (BA9) of Depressed Patients. Psychopharmacology 2014, 231, 3569–3580.
- Maguire, J. Neuroactive Steroids and GABAergic Involvement in the Neuroendocrine Dysfunction Associated with Major Depressive Disorder and Postpartum Depression. Front. Cell. Neurosci. 2019, 13, 83.
- Nappi, R.E.; Petraglia, F.; Luisi, S.; Polatti, F.; Farina, C.; Genazzani, A.R. Serum Allopregnanolone in Women with Postpartum “Blues”. Obstet. Gynecol. 2001, 97, 77–80.
- Zorumski, C.F.; Paul, S.M.; Izumi, Y.; Covey, D.F.; Mennerick, S. Neurosteroids, Stress and Depression: Potential Therapeutic Opportunities. Neurosci. Biobehav. Rev. 2013, 37, 109–122.
- Zorumski, C.F.; Paul, S.M.; Covey, D.F.; Mennerick, S. Neurosteroids as Novel Antidepressants and Anxiolytics: GABA-A Receptors and Beyond. Neurobiol. Stress 2019, 11, 100196.
- McEvoy, K.; Payne, J.L.; Osborne, L.M. Neuroactive Steroids and Perinatal Depression: A Review of Recent Literature. Curr. Psychiatry Rep. 2018, 20, 78.
- Osborne, L.M.; Gispen, F.; Sanyal, A.; Yenokyan, G.; Meilman, S.; Payne, J.L. Lower Allopregnanolone during Pregnancy Predicts Postpartum Depression: An Exploratory Study. Psychoneuroendocrinology 2017, 79, 116–121.
- Rasmusson, A.M.; Pinna, G.; Paliwal, P.; Weisman, D.; Gottschalk, C.; Charney, D.; Krystal, J.; Guidotti, A. Decreased Cerebrospinal Fluid Allopregnanolone Levels in Women with Posttraumatic Stress Disorder. Biol. Psychiatry 2006, 60, 704–713.
- Pinna, G. Allopregnanolone, the Neuromodulator Turned Therapeutic Agent: Thank You, Next? Front. Endocrinol. 2020, 11, 236.
- Pineles, S.L.; Nillni, Y.I.; Pinna, G.; Irvine, J.; Webb, A.; Arditte Hall, K.A.; Hauger, R.; Miller, M.W.; Resick, P.A.; Orr, S.P.; et al. PTSD in Women Is Associated with a Block in Conversion of Progesterone to the GABAergic Neurosteroids Allopregnanolone and Pregnanolone Measured in Plasma. Psychoneuroendocrinology 2018, 93, 133–141.
- Pineles, S.L.; Nillni, Y.I.; Pinna, G.; Webb, A.; Arditte Hall, K.A.; Fonda, J.R.; Irvine, J.; King, M.W.; Hauger, R.L.; Resick, P.A.; et al. Associations between PTSD-Related Extinction Retention Deficits in Women and Plasma Steroids That Modulate Brain GABAA and NMDA Receptor Activity. Neurobiol. Stress 2020, 13, 100225.
- Rasmusson, A.M.; King, M.W.; Valovski, I.; Gregor, K.; Scioli-Salter, E.; Pineles, S.L.; Hamouda, M.; Nillni, Y.I.; Anderson, G.M.; Pinna, G. Relationships between Cerebrospinal Fluid GABAergic Neurosteroid Levels and Symptom Severity in Men with PTSD. Psychoneuroendocrinology 2019, 102, 95–104.
- Cruz, D.A.; Glantz, L.A.; McGaughey, K.D.; Parke, G.; Shampine, L.J.; Kilts, J.D.; Naylor, J.C.; Marx, C.E.; Williamson, D.E. Neurosteroid Levels in the Orbital Frontal Cortex of Subjects with PTSD and Controls: A Preliminary Report. Chronic Stress 2019, 3, 247054701983857.
- Almeida, F.B.; Nin, M.S.; Barros, H.M.T. The Role of Allopregnanolone in Depressive-like Behaviors: Focus on Neurotrophic Proteins. Neurobiol. Stress 2020, 12, 100218.
- Locci, A.; Pinna, G. Neurosteroid Biosynthesis Down-Regulation and Changes in GABA A Receptor Subunit Composition: A Biomarker Axis in Stress-Induced Cognitive and Emotional Impairment: Neurosteroids and GABA: Biomarkers for Emotions. Br. J. Pharmacol. 2017, 174, 3226–3241.
- Pinna, G. Animal Models of PTSD: The Socially Isolated Mouse and the Biomarker Role of Allopregnanolone. Front. Behav. Neurosci. 2019, 13, 114.
- Caruso, D.; Pesaresi, M.; Abbiati, F.; Calabrese, D.; Giatti, S.; Garcia-Segura, L.M.; Melcangi, R.C. Comparison of Plasma and Cerebrospinal Fluid Levels of Neuroactive Steroids with Their Brain, Spinal Cord and Peripheral Nerve Levels in Male and Female Rats. Psychoneuroendocrinology 2013, 38, 2278–2290.
- Kancheva, R.; Hill, M.; Novák, Z.; Chrastina, J.; Velíková, M.; Kancheva, L.; Říha, I.; Stárka, L. Peripheral Neuroactive Steroids May Be as Good as the Steroids in the Cerebrospinal Fluid for the Diagnostics of CNS Disturbances. J. Steroid Biochem. Mol. Biol. 2010, 119, 35–44.
- Martin, J.; Plank, E.; Jungwirth, B.; Hapfelmeier, A.; Podtschaske, A.; Kagerbauer, S.M. Weak Correlations between Serum and Cerebrospinal Fluid Levels of Estradiol, Progesterone and Testosterone in Males. BMC Neurosci. 2019, 20, 53.
- Vining, R.F.; McGinley, R.A. The Measurement of Hormones in Saliva: Possibilities and Pitfalls. J. Steroid Biochem. 1987, 27, 81–94.
- Meulenberg, P.M.; Hofman, J.A. Salivary Progesterone Excellently Reflects Free and Total Progesterone in Plasma during Pregnancy. Clin. Chem. 1989, 35, 168–172.