The vaccination for the novel Coronavirus (COVID-19) is undergoing its final stages of analysis and testing. It is an impressive feat under the circumstances that we are on the verge of a potential breakthrough vaccination. This will help reduce the stress for millions of people around the globe, helping to restore worldwide normalcy. In this review, the analysis looks into how the new branch of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) came into the forefront of the world like a pandemic. This review will break down the details of what COVID-19 is, the viral family it belongs to and its background of how this family of viruses alters bodily functions by attacking vital human respiratory organs, the circulatory system, the central nervous system and the gastrointestinal tract.
1. Introduction
Covid-19 Breakdown and Background
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)—better known as Coronavirus or COVID-19—was first encountered in the capital city of the Hubei province of Wuhan, China. In late December 2019, health authorities began to identify unknown viral pneumonia cases that started to spread to other parts of China. Due to how much it had spread by early January 2020, an identification technique known as Reverse Transcription Polymerase Chain Reaction (RT-PCR) was applied, which enabled scientists in real-time to establish a diagnosis for these cases by way of distinguishing and isolating the novel Coronavirus from which viral pneumonia the patients were suffering was caused [1].
By the end of January 2020, after succumbing to considerable pressure the World Health Organization (WHO) officially declared COVID-19 as a pandemic, a Public Health Emergency of International Concern (PHEIC). Due to action that was not taken sooner in many parts of the world, the virus spread to around 25 countries by early February 2020. With the numbers increasing, guidelines and criteria for diagnosis, treatment and preventative measures had to be established rapidly [1][2]. Viral detection using RT-PCR identified the SARS-CoV-2 virus to be the disease which caused this viral transmission worldwide. This virus bore significant similarity to that found present within bats and was of the same family as Severe Acute Respiratory Syndrome Coronavirus 1 (SARS-CoV-1) and Middle East Respiratory Syndrome Coronavirus (MERS-CoV), therefore significantly narrowing down the likelihood that this had been somehow transmitted from bats to humans (owing to bats being the main reservoir for this virus). COVID-19 has a high recombinant and mutation rate due to its unique replication capabilities, enabling it to adapt to new host cells and different target sites [3]. So far, Covid-19 has been defined by 17 known mutations (14 non-synonymous mutations and 3 deletions), eight of these mutations have been on the spike protein, the main target site for the vaccination, with at least three of these mutations having a significant biological effect. These mutations, in particular N501Y, can incur a substantial change in the binding domain, resulting in enhancing the binding affinity to the human ACE2 enzyme. Another mutation (P681H) that is located directly close to the spike protein has shown the potential to increase infection and transmission. In terms of deletions that have occurred to the viral genome, the deletion of two amino acids has shown a link indicating immune escapability in immunocompromised patients thus enhancing viral infectivity [4]. The transmission of COVID-19 is from human-to-human contact [5], the most common infections occur from sufferers who are asymptomatic, therefore transmitting the virus without being aware they are carriers [6].
By the end of January 2020, after succumbing to considerable pressure the World Health Organization (WHO) officially declared COVID-19 as a pandemic, a Public Health Emergency of International Concern (PHEIC). Due to action that was not taken sooner in many parts of the world, the virus spread to around 25 countries by early February 2020. With the numbers increasing, guidelines and criteria for diagnosis, treatment and preventative measures had to be established rapidly [1,2]. Viral detection using RT-PCR identified the SARS-CoV-2 virus to be the disease which caused this viral transmission worldwide. This virus bore significant similarity to that found present within bats and was of the same family as Severe Acute Respiratory Syndrome Coronavirus 1 (SARS-CoV-1) and Middle East Respiratory Syndrome Coronavirus (MERS-CoV), therefore significantly narrowing down the likelihood that this had been somehow transmitted from bats to humans (owing to bats being the main reservoir for this virus). COVID-19 has a high recombinant and mutation rate due to its unique replication capabilities, enabling it to adapt to new host cells and different target sites [3]. So far, Covid-19 has been defined by 17 known mutations (14 non-synonymous mutations and 3 deletions), eight of these mutations have been on the spike protein, the main target site for the vaccination, with at least three of these mutations having a significant biological effect. These mutations, in particular N501Y, can incur a substantial change in the binding domain, resulting in enhancing the binding affinity to the human ACE2 enzyme. Another mutation (P681H) that is located directly close to the spike protein has shown the potential to increase infection and transmission. In terms of deletions that have occurred to the viral genome, the deletion of two amino acids has shown a link indicating immune escapability in immunocompromised patients thus enhancing viral infectivity [4]. The transmission of COVID-19 is from human-to-human contact [5], the most common infections occur from sufferers who are asymptomatic, therefore transmitting the virus without being aware they are carriers [6].
Symptoms of COVID-19 as was mentioned consist of two states, the (i) symptomatic state and the (ii) asymptomatic state. The symptomatic state can be easily noticed through the patient showing multiple different symptoms, one of them being the Acute Respiratory Disease Syndrome (ARDS), which include fever, cough, tiredness, sore throat, headache, and myalgia. More severe symptoms include aches and pains, diarrhoea, conjunctivitis, loss of taste and smell, a rash on skin and discoloration of fingers or toes; the most severe cases include difficulty breathing, chest pains or pressure and can even lead to loss of speech and movement [7]—some of the symptoms can result in multiple organ failure and eventually death. ARDS patients who experience symptoms tend to carry underlying health conditions, a suppressed immune system or are of older age. According to the literature, asymptomatic COVID-19 sufferers are the main source of transmission; through their respiratory droplets being airborne, as well as transmitted through virus-contaminated containers and foods [8][9]. Asymptomatic carriers show no symptoms of the virus due to an immune system capable of combatting the virus. However, they are capable of infecting others, henceforth making the virus capable of spreading around and becoming sometimes untraceable. The only way to identify an asymptomatic patient is through the administration of an RT-PCR. This, therefore, makes it difficult for countries to conduct identification tests whilst attempting to control the spread of the virus [10].
Symptoms of COVID-19 as was mentioned consist of two states, the (i) symptomatic state and the (ii) asymptomatic state. The symptomatic state can be easily noticed through the patient showing multiple different symptoms, one of them being the Acute Respiratory Disease Syndrome (ARDS), which include fever, cough, tiredness, sore throat, headache, and myalgia. More severe symptoms include aches and pains, diarrhoea, conjunctivitis, loss of taste and smell, a rash on skin and discoloration of fingers or toes; the most severe cases include difficulty breathing, chest pains or pressure and can even lead to loss of speech and movement [7]—some of the symptoms can result in multiple organ failure and eventually death. ARDS patients who experience symptoms tend to carry underlying health conditions, a suppressed immune system or are of older age. According to the literature, asymptomatic COVID-19 sufferers are the main source of transmission; through their respiratory droplets being airborne, as well as transmitted through virus-contaminated containers and foods [8,9]. Asymptomatic carriers show no symptoms of the virus due to an immune system capable of combatting the virus. However, they are capable of infecting others, henceforth making the virus capable of spreading around and becoming sometimes untraceable. The only way to identify an asymptomatic patient is through the administration of an RT-PCR. This, therefore, makes it difficult for countries to conduct identification tests whilst attempting to control the spread of the virus [10].
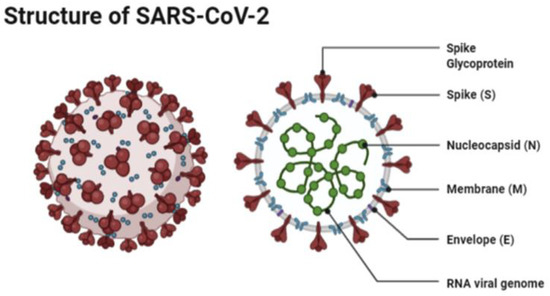
The structure of SARS-CoV-2 has shown to have a single strand enveloped RNA (sRNA). The size of the virus is between 50 and 150 nm in diameter, its linearity and positive-sense RNA genome is large. It belongs to the CoV-2 family, which was firstly found in the mid 1960s [11][12]. This family of viruses has a spherical shape, with envelopes containing helical nucleo-capsids and nucleoproteins; these are associated with the genomic structure of RNAs. The virus is capable of attaching itself to the host cells of its target due to a trimer of spike glycol-proteins, which include hemagglutinin esterase; there are also integral membrane and envelope proteins [13]. The virus can infect humans. Although animals will have varying immune responses to the virus, their immune system is more equipped to combat it and therefore it does not spread between animals as quickly as it does between humans. The virus targets the respiratory, hepatic, gastro-intestinal and neurological systems [12]. The name Coronavirus is due to the presence of the crown-like structures identified when scanned under the electron microscope. The viral structure consists of envelopes that contain helical nucleo-capsids and nucleoproteins (N), these are associated with the RNA genome. Embedded in the envelope is a 2 nm trimer of spike glycoproteins (S), this is the main source of the virus’s attachment to the receptor of the host cells. Within the virus, it also consists of integral membranes (M) and envelope proteins (E). Beta-Coronaviruses have an additional membrane glycoprotein named hemagglutinin esterase, which contains 5–7 nm long spikes (See
The structure of SARS-CoV-2 has shown to have a single strand enveloped RNA (sRNA). The size of the virus is between 50 and 150 nm in diameter, its linearity and positive-sense RNA genome is large. It belongs to the CoV-2 family, which was firstly found in the mid 1960s [11,12]. This family of viruses has a spherical shape, with envelopes containing helical nucleo-capsids and nucleoproteins; these are associated with the genomic structure of RNAs. The virus is capable of attaching itself to the host cells of its target due to a trimer of spike glycol-proteins, which include hemagglutinin esterase; there are also integral membrane and envelope proteins [13]. The virus can infect humans. Although animals will have varying immune responses to the virus, their immune system is more equipped to combat it and therefore it does not spread between animals as quickly as it does between humans. The virus targets the respiratory, hepatic, gastro-intestinal and neurological systems [12]. The name Coronavirus is due to the presence of the crown-like structures identified when scanned under the electron microscope. The viral structure consists of envelopes that contain helical nucleo-capsids and nucleoproteins (N), these are associated with the RNA genome. Embedded in the envelope is a 2 nm trimer of spike glycoproteins (S), this is the main source of the virus’s attachment to the receptor of the host cells. Within the virus, it also consists of integral membranes (M) and envelope proteins (E). Beta-Coronaviruses have an additional membrane glycoprotein named hemagglutinin esterase, which contains 5–7 nm long spikes (See ). There are different families of the Coronavirus; the International Committee on Taxonomy of Viruses (ICTV) has separated them into different genres depending on their activity and structure. The genres are named; Alpha, Beta, Gamma and Delta Coronaviruses [14]. Several human Coronaviruses (alpha-CoVs, HCoVs-NL63, beta-CoVs, HCoVs-OC43, HCoVs-229E, HCoVs-HKU1, MERS-CoV, SARS-CoV and ARDS have been identified [15]. New versions of the Coronavirus will appear due to the large genomic potential, rapid mutation capabilities, high prevalence and wide distribution within the bird and animal kingdom. The emergence of CoVs is due to birds being able to carry this viral form and transfer it from area to area through flying and being capable of inhabiting in between groups [16][17].
). There are different families of the Coronavirus; the International Committee on Taxonomy of Viruses (ICTV) has separated them into different genres depending on their activity and structure. The genres are named; Alpha, Beta, Gamma and Delta Coronaviruses [14]. Several human Coronaviruses (alpha-CoVs, HCoVs-NL63, beta-CoVs, HCoVs-OC43, HCoVs-229E, HCoVs-HKU1, MERS-CoV, SARS-CoV and ARDS have been identified [15]. New versions of the Coronavirus will appear due to the large genomic potential, rapid mutation capabilities, high prevalence and wide distribution within the bird and animal kingdom. The emergence of CoVs is due to birds being able to carry this viral form and transfer it from area to area through flying and being capable of inhabiting in between groups [16,17].
Figure 1.
The structure of SAR-CoV-2 under a microscope as illustrated by Agarwal et al. [18].
SARS-CoV-2 binds to the host cell Angiotensin-Converting Enzyme 2 (ACE 2) through its spike proteins. These spike proteins consist of two subunits, the receptor-binding subunit, which facilitates binding of the virus to the host cell, and the membrane fusing subunit, which allows for the fusing of the membranes of the virus and host cell. Once the virus binds to the host cell, the viral molecule then enters into the host cell [19]. Prior to entering the cells, the protease enzyme TMPRSS2 activates the spike proteins. The combination of activation and binding to ACE2 are required for successful admission into the cell [20]. Once entered they translate small parts of the virus onto non-structural proteins. The proteins then form an enzyme called RNA Dependent RNA Polymerase. The enzyme induces a double membrane vesicle, through the restructuring of the endoplasmic reticulum of the cell. Once these vesicles are formed, continuous replication and transcription are made of the sRNA gene coding for SARS-CoV-2 [21]. Once this is complete all the viral proteins with the sRNA are collated in the endoplasmic reticulum and the Golgi apparatus of the cell, hence forming the new virus particle. These viruses then release and spread within the body, attacking target sites as mentioned above [22].
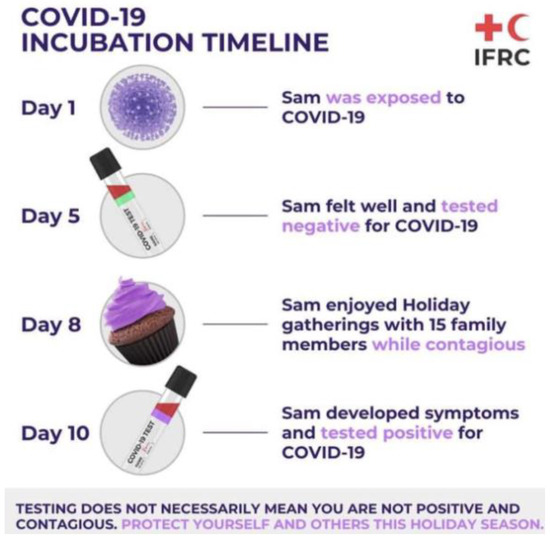
The most common test to detect the virus is through RT-PCR. This test requires both a nasal and throat swab. The test detects the RNA of the virus, which may be present within a patient prior to the formation of antibodies or symptoms. With this test, early-stage detection can be achieved. The RT-PCR targets two parts to the virus, the Open Reading Frame Gene (ORFG) and the viral nucleo-capsid regions. The test works through the reverse transcription of the RNA of the virus into a complementary DNA (cDNA) [21]. This is then amplified in the Real-Time Polymerase Chain Reaction thermal cycle. The dye used produces fluorescent signals, whereby the RT-PCR is then capable of automatically forming a curve, thus giving a quantitative analysis for the presence of the SARS-CoV-2 virus at the nucleic acid level. RT-PCR can detect the virus in asymptomatic persons, however, the test is capable of giving false-negatives hence patients may be tested twice before being confirmed as positive or negative [21][23]. The RT-PCR kit remains an effective kit to use in the identification of SAR-CoV-2, however, one of the main worries for challenges arising from this analysis is that cases may have gone undetected; several studies have shown that the clinical sensitivity of the analysis of respiratory swabs was at around 70% effective. This was due to the timing of these swabs, the type of specimen obtained and the quality of the sample taken. The viruses present in the upper respiratory tract for the first several days following the onset of symptoms, hence after 5 days of symptoms, it becomes increasingly difficult to identify the virus via RT-PCR. In the latter stages, for correct and accurate reading, swabs from the lower respiratory tract will yield a higher rate of detection. Due to these nuances, it has been challenging laboratory professionals to truly define the clinical sensitivity of SARS-CoV-2 real-time PCR and has required that negative results be interpreted in the context of the timing of the sample [24]. An example of problems faced for SAR-CoV-2 RT-PCR testing is shown in
The most common test to detect the virus is through RT-PCR. This test requires both a nasal and throat swab. The test detects the RNA of the virus, which may be present within a patient prior to the formation of antibodies or symptoms. With this test, early-stage detection can be achieved. The RT-PCR targets two parts to the virus, the Open Reading Frame Gene (ORFG) and the viral nucleo-capsid regions. The test works through the reverse transcription of the RNA of the virus into a complementary DNA (cDNA) [21]. This is then amplified in the Real-Time Polymerase Chain Reaction thermal cycle. The dye used produces fluorescent signals, whereby the RT-PCR is then capable of automatically forming a curve, thus giving a quantitative analysis for the presence of the SARS-CoV-2 virus at the nucleic acid level. RT-PCR can detect the virus in asymptomatic persons, however, the test is capable of giving false-negatives hence patients may be tested twice before being confirmed as positive or negative [21,23]. The RT-PCR kit remains an effective kit to use in the identification of SAR-CoV-2, however, one of the main worries for challenges arising from this analysis is that cases may have gone undetected; several studies have shown that the clinical sensitivity of the analysis of respiratory swabs was at around 70% effective. This was due to the timing of these swabs, the type of specimen obtained and the quality of the sample taken. The viruses present in the upper respiratory tract for the first several days following the onset of symptoms, hence after 5 days of symptoms, it becomes increasingly difficult to identify the virus via RT-PCR. In the latter stages, for correct and accurate reading, swabs from the lower respiratory tract will yield a higher rate of detection. Due to these nuances, it has been challenging laboratory professionals to truly define the clinical sensitivity of SARS-CoV-2 real-time PCR and has required that negative results be interpreted in the context of the timing of the sample [24]. An example of problems faced for SAR-CoV-2 RT-PCR testing is shown in .
Figure 2.
Illustration of a potential problem that is faced with RT-PCR testing due to difficulties in early detection of SAR-CoV-2 as described by the Red Cross [25].
2. The Research and History Ortho-Coronavirinae
Coronaviruses constitute the subfamily
Ortho-Coronavirinae.
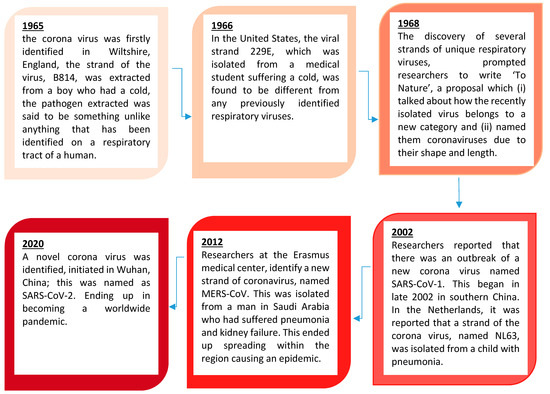
In this section, we look at the history of this viral family, the research that has been undertaken and what we know about the coronaviruses.
shows the history of the coronaviruses.
Figure 3.
Brief timeline history of the Ortho-Coronavirinae as illustrated by Williams 2020 [26].