Oxidative stress may be defined as an imbalance between reactive oxygen species (ROS) and the antioxidant system to counteract or detoxify these potentially damaging molecules. This phenomenon is a common feature of many human disorders such as cardiovascular disease. Many of the risk factors, including smoking, hypertension, hypercholesterolemia, diabetes, and obesity are associated with an increased risk of developing cardiovascular disease involving an elevated oxidative stress burden, either due to enhanced ROS production or decreased antioxidant protection. There is a number of therapeutic options to treat oxidative stress-associated cardiovascular diseases. Numerous studies have focused on the utility of antioxidant supplementation. However, whether antioxidant supplementation has any preventive and/or therapeutic value in cardiovascular pathology is still a matter of debate.
Oxidative stress may be defined as an imbalance between reactive oxygen species (ROS) and the antioxidant system to counteract or detoxify these potentially damaging molecules. This phenomenon is a common feature of many human disorders such as cardiovascular disease. Many of the risk factors, including smoking, hypertension, hypercholesterolemia, diabetes, and obesity are associated with an increased risk of developing cardiovascular disease involving an elevated oxidative stress burden, either due to enhanced ROS production or decreased antioxidant protection. There is a number of therapeutic options to treat oxidative stress-associated cardiovascular diseases. Numerous studies have focused on the utility of antioxidant supplementation. However, whether antioxidant supplementation has any preventive and/or therapeutic value in cardiovascular pathology is still a matter of debate.
- oxidative stress
- cardiovascular disease
1. Introduction
Oxidative stress has an important role in the onset and in the progression of several diseases, and in particular, in cardiovascular diseases. Oxidative stress is caused by the overproduction of reactive oxygen species (ROS), which include both the free radicals and their non-radical intermediates, such as superoxide anion (O2•−), hydroxyl ion (OH•), hydrogen peroxide (H2O2), and peroxyl radicals (ROO•), alkoxyl (RO•), singlet oxygen (1O2), and ozone (O3). The burst of ROS is associated with an imbalance between the generated ROS and the antioxidant defense systems. Evidence shows that oxidative stress plays an important role in the progression of various cardiovascular diseases, such as atherosclerosis, heart failure (HF), cardiac arrhythmia, and myocardial ischemia-reperfusion (I/R) injury. A lot of work has been devoted to the studies of antioxidants therapies in the prevention and treatment of these cardiovascular diseases. While some clinical trials have shown positive results, others are controversial. This is partly due to the incorrect evaluation of biomarkers of oxidative stress, and in particular, to the lack of the assessment of key ROS-producing enzymes, such as NADPH oxidase. Furthermore, the choice of the dosage and type of antioxidant used in the treatment or prevention of cardiovascular diseases is not accurate and specific for each pathology.
2. Biomarkers of Oxidative Stress in patients with cardiovascular risk factors
Reactive oxygen species (ROS) are key cellular components that play an important role in various physiological conditions, as well as in the development of several diseases [1]. The ROS play a dual role, both beneficial and toxic to the organism. At moderate or low levels, ROS have beneficial effects and act on various physiological functions like immune function (i.e., defense against pathogenic microorganisms), in some number of intracellular pathways, and in redox regulation. Conversely, high concentrations of ROS induce oxidative stress, a pathological condition characterized by an overload of free radicals that are not neutralized, have a toxic effect, and modify the integrity of cell membranes and other structures, such as organic macromolecules [2]. Oxidative stress is responsible for numerous chronic and degenerative diseases, such as cancer, autoimmune disorders, rheumatoid arthritis, aging, neurodegenerative and cardiovascular diseases. Considering the evidence about the association between oxidative stress with a multitude of human diseases, the measurement of oxidative stress biomarkers plays a pivotal role in the evaluation of the health status, as well as the development of oxidative stress-mediated disorders [3].
Although many biomarkers can be used to determine oxidative stress, most of these benchmarks are limited in vivo [4][5]. The precise measurement of ROS in vascular cells and tissues represents a challenge because of their low levels and transient lifetimes [6]. Indeed, when produced within living cells, the short half-life (seconds) of a certain ROS limits the distance it can diffuse and thereby its radius of action. This means that the direct reaction of a short-lived ROS like O2•− in situ is likely restricted to a small sub-cellular volume surrounding the site of its generation (“local” ROS), whereas ROS with a longer half-life like H2O2 might be more suited for global signaling [7].
Malondialdehyde (MDA) and thiobarbituric acid reactive substances (TBARS) are commonly used to evaluate lipid peroxidation products along with 4-hydroxy-2-nonenal (4-HNE), conjugated dienes (CD), lipid hydroperoxides (LOOH), and 8-isoprostaglandin F2α (8-iso-PGF2α), produced by arachidonic acid peroxidation, that was introduced to evaluate lipid peroxidation in biological fluids samples (e.g., urine and plasma) [8]. Moreover, several methods have been developed to evaluate protein oxidative modification, such as determining advanced oxidation protein products (AOPP) [9][10]. Besides lipids and proteins, even DNA double strands undergo chemical modification that can determine genetic damages on the daughter strands [11]. Finally, analysis of oxidative stress has been carried out estimating levels and activities of enzymatic and non-enzymatic antioxidants in biological samples, such as plasma, serum, and tissue samples. More specifically, superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx) and glutathione S-Transferase (GSTs), H2O2 breakdown activity (HBA) are taken into account in the determination of enzymatic antioxidant status [12]
Recently, there is attention to the validation of new biomarkers of oxidative stress, as they have a potential application in clinical practice. According to the World Health Organization, a biomarker is “any substance structure or process that can be measured in the body or its products and influence or predict the incidence of outcome or disease”. Among the biomarkers of oxidative stress used in the clinical practice, we can find: Advanced Glycation End Products (AGEs), Oxidized Low-Density Lipoprotein (oxLDL), Protein Oxidation Is Advanced Oxidation Protein Products (AOPP), Lipid Oxidation Products, 8-Hydroxy-2′-Deoxyguanosine (8-OHdG), Hydrogen Peroxide (H2O2) and NOX2 Activity (sNOX2-dp).
Cardiovascular Disease (CVD) is worldwide known to be a major cause of death and comorbidity. Atherosclerosis is the key pathophysiological mechanism underlying the development of CVD. In particular, atherosclerosis, a chronic inflammation that affects arteries, may remain clinically undetected for many years before an acute event such as Ischemic Heart Disease (IHD) or a stroke and Peripheral Vascular Disease (PVD) [13]. CVDs are caused by multiple factors that can be divided into un-modifiable and modifiable risk factors. Age, gender, family history and ethnicity are all un-modifiable because the individual can do nothing to avoid these risk factors.
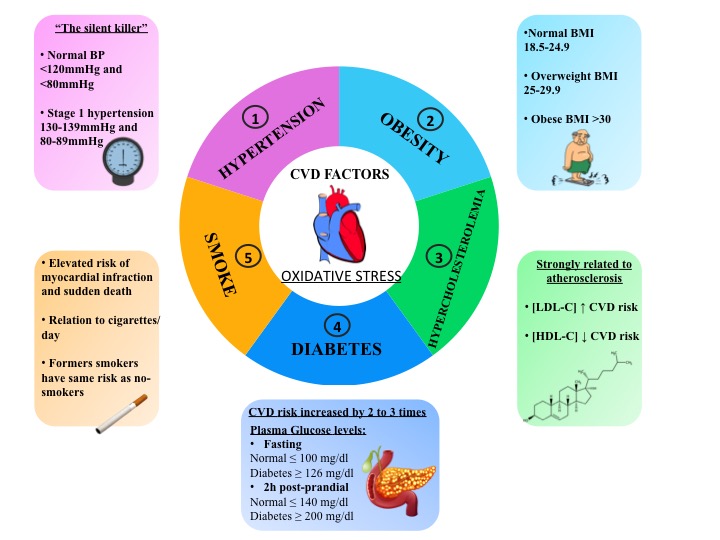
Though the characteristics of un-modifiable risk are greatly suitable for risk stratification, the modifiable factors have the advantage of being a possible target for pharmaceutical intervention in order to lower cardiovascular risks. Among the main modifiable traditional cardiovascular risk factors, there are hypertension, diabetes mellitus, obesity, hypercholesterolemia and smoking (Figure 1). Furthermore, these cardiovascular risk factors are associated with increased production of oxidative stress (Figure 1). Clinical human studies have supported the association between oxidative stress and cardiovascular events and different types of molecular biomarkers provide a powerful approach to the understanding of cardiovascular risk factors with consequent applications in epidemiology and clinical studies and in the prevention, diagnosis and management of cardiovascular diseases.
3. Antioxidant supplementation in patients with cardiovascular risk factors
Many clinical studies demonstrated the beneficial effect of antioxidant supplementation in the suppression of oxidative stress-mediated damage in patients with cardiovascular risk factors.
The term “antioxidants” defines chemical substances that slow down the damage caused by oxygen to organisms. Antioxidants are one of the mechanisms that the body uses to fight against oxidative stress with the role to balance the negative effects of oxidant agents and protect cells from oxidative damage [2]. We can identify two macro groups of antioxidants: those that are produced by the body itself (i.e. endogenous antioxidants) and those that derive from dietary sources (i.e. exogenous antioxidants). Endogenous antioxidants can be divided into two classes: enzymatic and non-enzymatic antioxidants. Some enzymatic antioxidants are catalase (CAT) that degrades hydrogen peroxide (H2O2) to water and oxygen, glutathione reductase (GRx), glutathione peroxidase (GPx) that catalyzes the reduction of H2O2 by the reduced form of glutathione (GSH) creating a glutathione bridge with another glutathione molecule (GSSG), and superoxide dismutase (SOD) that catalyzes the dismutation of superoxide anion radical (O2-) into H2O2 and oxygen (O2) [14].
The non-enzymatic antioxidants include nutrients that are not produced by the body and thus need to be included through the diet. Nutrient antioxidants are contained in fruits, vegetables and, fishes and are extremely important because each one of them has a role in oxidative stress neutralization [15]. According to their role in reducing oxidative stress-mediated cardiovascular risk, these exogenous molecules can represent a useful tool in clinical practice [16]. Specifically, natural extracts, such as polyphenols, exert an antioxidant activity that includes suppression of ROS formation by either inhibition of enzymes involved in their production, like NOX2 [17], scavenging of ROS [18], or up-regulation or protection of antioxidant defences [19].
The most widely used antioxidants in clinical practice include vitamins E and C; omega-3 fatty acids including eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and alpha-linolenic acid (ALA) and omega-6 fatty acids; polyphenols including flavonoids and non-flavonoids; selenium; lipoic acid; coenzyme Q10. The choice of the type of antioxidant supplementation that best affects cardiovascular disease is still a challenge. The results of several antioxidant supplementations in different cardiovascular diseases are disparately ranging from possibly beneficial to many futile to some harmful effects.
3.1 HYPERTENSION
The pathophysiology of hypertension involves a complex interaction of multiple vascular effectors including the activation of the sympathetic nervous system, of the renin-angiotensin-aldosterone system, and the inflammatory mediators. Oxidative stress and endothelial dysfunction are consistently observed in hypertensive subjects and have a causal role in the molecular processes leading to hypertension.
The antioxidant vitamins C and E supplementation resulted in a reduction in blood pressure, oxidative stress biomarkers, and increased fluidity by PUFA proportion in the membrane [20]. Conversely, high-dose vitamin C and vitamin E failed to prevent methionine-induced homocysteinemia reduction of endothelium-dependent dilation despite the reduction of peroxidation in hypertensive patients [21].
Quercetin but not epicatechin decreased the plasma concentration of methylglyoxal, which is a reactive di-carbonyl intermediate and a precursor of advanced glycation end-products [22]. On the same line, epicatechin supplementation improves FMD but not statistically significant compared to placebo [23]. In mildly hypertensive patients, coenzyme Q10 supplementation was effective in decreasing some pro-inflammatory factors, such as IL6 and hs-CRP. Adjunctive coenzyme Q10 therapy was not associated with statistically significant reductions in systolic or diastolic blood pressure or heart rate [24].
3.2 DIABETES
As oxidative stress plays a key role in the development and the progression of diabetes and its related complications, several antioxidant supplementations were tested.
The supplementation with antioxidant vitamins in diabetic patients exerts beneficial effects that could improve the clinical condition and attenuate or prevent diabetic pathogenesis and complications that, secondly to poor glycemic control, could attribute to the imbalance between the decline in the endogenous antioxidants and increasing production of the ROS. Indeed, Vitamin C or Vitamin E supplementation improves fasting blood sugar, lipid profile, insulin, homeostasis model assessment of insulin resistance (HOMA-IR) and then increases the antioxidant profile and reduces oxidative biomarkers [25][26][27]. However, other studies do not support the beneficial effect of vitamin supplementation. For example, no differences were seen in the endothelial function measurement and total plasma antioxidant capacity (TAOC) before and after combined vitamin C and E therapy [28] or in plasma oxyphytosterol concentrations and other oxidative biomarkers [29].
The antioxidant effects of resveratrol supplementation in attenuating the increased oxidative stress in diabetes mellitus patients have been investigated in several studies [30][31][32][33][34].
Finally, several studies evaluated the effect of a supplement containing lipoic acid on glyco-metabolic control and oxidative stress markers. These studies demonstrated that oral supplementation of alpha-lipoic acid reduces fasting plasma glucose, glycated hemoglobin (HbA1c) and fasting plasma insulin with an improvement of lipid profile. The antioxidant effect resulted in an increase of SOD, and GSH-Px, and a decrease of MDA [35][36].
3.3 HYPERCHOLESTEROLEMIA
The interaction of the combination of statins with n-3 fatty acids on oxidative stress was evaluated in hypercholesterolemic women receiving a mixture of EPA and DHA. Results showed that statins and n-3 fatty acids increased oxidative stress as a result of increased plasma malondialdehyde and SOD activity whereas reduced catalase expression [37]. Accordingly, administration of N-3 fatty acids to patients treated with statins has no effect on oxidative stress parameter, that is STAT-8-Isoprostane, and on endothelial function. However, a combination of statins and N-3 fatty acid inhibits platelet aggregation, alters inflammatory status, and positively affects daytime blood pressure [38].
Only one study evaluated the effect of resveratrol in hypercholesterolemic patients. In these patients, with a higher demand for antioxidant activity due to higher cholesterol levels, resveratrol consumption significantly increased Vitamin E levels without changes in TAC or in total cholesterol levels [39].
3.4 OBESITY
Vitamins (E and C) supplementation improved antioxidant-oxidant balance by increasing antioxidant status and reducing oxidative stress biomarkers, namely F(2)-isoprostanes and F(2)-isoprostane metabolites, but did not affect the inflammatory markers measured [40]. Vitamin C intravenous infusions in overweight or obese grade I subjects reduced protein carbonylation, one of the most harmful irreversible oxidative protein modifications, and a major hallmark of oxidative stress-related disorders [41]. Conjugated linoleic acid supplementation plus Vitamin E improved insulin resistance, lipid disturbances, oxidative stress as total antioxidant capacity increased and MDA significantly decreased in obese patients with NAFLD [42].
Resveratrol supplemented to obese subjects significantly decreased expression of pathways related to energy metabolism, oxidative stress, inflammation [43] and maintains healthy circulatory function as indicated by a 23% increase in FMD compared to placebo [44].
3.4 SMOKE
The short-term Vitamin E-rich supplementation in combination with smoking cessation improved vascular endothelial function as indicated by increased brachial artery flow-mediated dilation (FMD) by 1.3 %. Moreover, the pro-inflammatory levels of mediators such as TNF-α and myeloperoxidase decreased after γ-T-rich supplements and these were inversely related to FMD. However, the supplementation doesn’t affect plasma oxidized LDL and urinary F2-isoprostanes [45][46]. Long-term supplementation with vitamin E (36 months) lowered urine 8-iso-prostaglandin F2-alpha (8-iso-PGF2α) by 21% [47].
Omega-3 fatty acid supplementation for 3 months decreases total oxidant status and oxidative stress index [48]. Oral supplementation of resveratrol for 30 days significantly reduced C-reactive protein (CRP) and triglyceride concentrations and increased Total Antioxidant Status (TAS) values [49].
4. Concluding remarks
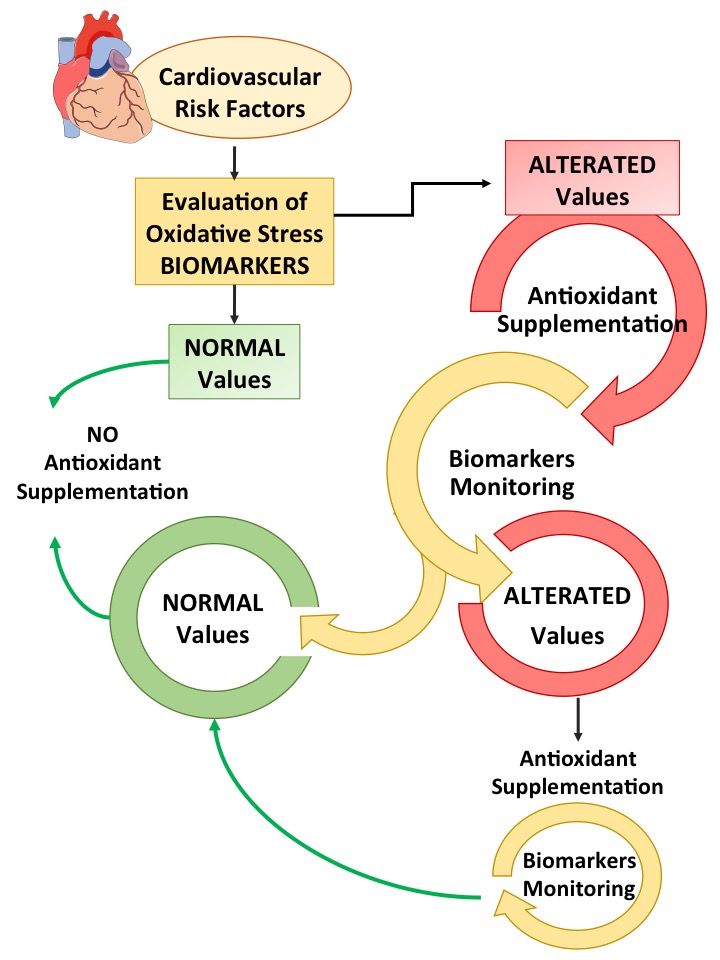
To date, the role of oxidative stress in the onset and progression of atherosclerosis and its impact on the development of cardiovascular events has been widely described. Several studies demonstrated an increase of biomarkers of oxidative stress in the setting of cardiovascular disease and outcome. Even if the suppression of oxidative stress using antioxidants is beneficial as reported by many clinical studies, the effectiveness of antioxidant therapies is controversial for several reasons. First of all, in different stages of diseases, oxidative stress may have different roles according to the oxidative stress levels. Thus, it is crucial to verify oxidative stress biomarkers levels to give the antioxidant treatments at the appropriate time. For example, researchers or clinicians should focus on the antioxidant power of a patient rather than looking for a particular antioxidant. Among the methods that evaluate this power, HBA is a promising new method that is able to evaluate the ability of each individual to neutralize H2O2, a cell-permeable ROS generated by cellular metabolism involved in intracellular signaling, that exerts a strong impact on cardiovascular pathophysiology. Indeed, in patients with atrial fibrillation, a reduced ability to scavenge H2O2, as indicated by reduced serum HBA, predicted cardiovascular events [12]. Moreover, oxidative stress should be assessed with methods that evaluate more the activity of the enzymes involved in the production of ROS than molecules produced by oxidative stress. Among these methods, the evaluation of NOX2 activity seems to correlate well with the severity of cardiovascular diseases and also with cardiovascular events. Second, not all antioxidants are effective in modifying the outcome of cardiovascular risk factors and the dosing strategies in clinical trials are different, even in the same pathology. Finally, not all biomarkers of oxidative stress are useful for monitoring the clinical outcome of cardiovascular risk factors. Taken together, this information highlighted that antioxidant therapy must be considered to all intents and purposes a pharmacological therapy and therefore that is extremely important to monitor the dosage and time of administration as suggested in Figure 2.
References
- Alugoju Phaniendra; Dinesh Babu Jestadi; Latha Periyasamy; Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian Journal of Clinical Biochemistry 2014, 30, 11-26, 10.1007/s12291-014-0446-0.
- Marian Valko; Dieter Leibfritz; Jan Moncol; Mark T.D. Cronin; Milan Mazur; Joshua Telser; Free radicals and antioxidants in normal physiological functions and human disease. The International Journal of Biochemistry & Cell Biology 2007, 39, 44-84, 10.1016/j.biocel.2006.07.001.
- Meghri Katerji; Maria Filippova; Penelope Duerksen-Hughes; Approaches and Methods to Measure Oxidative Stress in Clinical Samples: Research Applications in the Cancer Field. Oxidative Medicine and Cellular Longevity 2019, 2019, 1-29, 10.1155/2019/1279250.
- Jonathan Arauz; Erika Ramos-Tovar; Pablo Muriel; Redox state and methods to evaluate oxidative stress in liver damage: From bench to bedside.. Annals of Hepatology 2016, 15, 160–173, 10.5604/16652681.1193701.
- Keiki Ogino; Da-Hong Wang; Biomarkers of oxidative/nitrosative stress: an approach to disease prevention.. Acta Med. Okayama 2007, 61, 181-189.
- Sergey I. Dikalov; Rafal R. Nazarewicz; Measurements of Reactive Oxygen Species in Cardiovascular Studies. Systems Biology of Free Radicals and Antioxidants 2014, 49, 1435-1450, 10.1007/978-3-642-30018-9_45.
- Marleen Forkink; Jan A.M. Smeitink; Roland Brock; Peter H.G.M. Willems; Werner J.H. Koopman; Detection and manipulation of mitochondrial reactive oxygen species in mammalian cells. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 2010, 1797, 1034-1044, 10.1016/j.bbabio.2010.01.022.
- Rui Yu; Guiqing Zhao; John W. Christman; Lei Xiao; Richard B. Van Breemen; Method Development and Validation for Ultra-High Pressure Liquid Chromatography/Tandem Mass Spectrometry Determination of Multiple Prostanoids in Biological Samples. Journal of AOAC International 2013, 96, 67-76, 10.5740/jaoacint.12-280.
- Liang-Jun Yan; Michael J. Forster; Chemical probes for analysis of carbonylated proteins: A review. Journal of Chromatography B 2011, 879, 1308-1315, 10.1016/j.jchromb.2010.08.004.
- Elisabetta Bigagli; Maura Lodovici; Circulating Oxidative Stress Biomarkers in Clinical Studies on Type 2 Diabetes and Its Complications. Oxidative Medicine and Cellular Longevity 2019, 2019, 1-17, 10.1155/2019/5953685.
- Georgia-Persephoni Voulgaridou; Ioannis Anestopoulos; Rodrigo Franco; Mihalis I. Panayiotidis; Aglaia Pappa; DNA damage induced by endogenous aldehydes: Current state of knowledge. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 2011, 711, 13-27, 10.1016/j.mrfmmm.2011.03.006.
- Roberto Carnevale; Cristina Nocella; Pasquale Pignatelli; Simona Bartimoccia; Lucia Stefanini; Stefania Basili; Marta Novo; Alessandra D'amico; Vittoria Cammisotto; Daniele Pastori; et al.Francesco Violi Blood hydrogen peroxide break-down activity in healthy subjects and in patients at risk of cardiovascular events. Atherosclerosis 2018, 274, 29-34, 10.1016/j.atherosclerosis.2018.04.025.
- Piepoli, M. F.; Hoes, A. W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A. L.; Cooney, M.-T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al.Graham, I.Hall, M. S.Hobbs, F. D. R.Løchen, M.-L.Löllgen, H.Marques-Vidal, P.Perk, J.Prescott, E.Redon, J.Richter, D. J.Sattar, N.Smulders, Y.Tiberi, M.van der Worp, H. B.van Dis, I.Verschuren, W. M. M.Binno, S. ESC Scientific Document Group 2016 European Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J 2016, 37, 2315–2381, doi:10.1186/s12889-018-5263-6..
- Silvana Balzan Valter Lubrano; Silvana Balzan; Enzymatic antioxidant system in vascular inflammation and coronary artery disease. World Journal of Experimental Medicine 2015, 5, 218-224, 10.5493/wjem.v5.i4.218.
- Aurelia Magdalena Pisoschi; Aneta Pop; The role of antioxidants in the chemistry of oxidative stress: A review. European Journal of Medicinal Chemistry 2015, 97, 55-74, 10.1016/j.ejmech.2015.04.040.
- Cristina Nocella; Vittoria Cammisotto; Fabio Pigozzi; Paolo Borrione; Chiara Fossati; Alessandra D’Amico; Roberto Cangemi; Mariangela Peruzzi; Giuliana Gobbi; Evaristo Ettorre; et al.Giacomo FratiElena CavarrettaRoberto CarnevaleSMiLe Group Impairment between Oxidant and Antioxidant Systems: Short- and Long-term Implications for Athletes' Health.. Nutrients 2019, 11, 1353, 10.3390/nu11061353.
- R. Carnevale; L. Loffredo; P. Pignatelli; C. Nocella; S. Bartimoccia; S. Di Santo; F. Martino; E. Catasca; L. Perri; Francesco Violi; et al. Dark chocolate inhibits platelet isoprostanes via NOX2 down-regulation in smokers. Journal of Thrombosis and Haemostasis 2012, 10, 125-132, 10.1111/j.1538-7836.2011.04558.x.
- Hassan Y. Aboul-Enein; Irena Kruk; Aleksandra Kładna; Krzysztof Lichszteld; Teresa Michalska; Scavenging effects of phenolic compounds on reactive oxygen species. Biopolymers 2007, 86, 222-230, 10.1002/bip.20725.
- Yunbo Li; Zhuoxiao Cao; Hong Zhu; Upregulation of endogenous antioxidants and phase 2 enzymes by the red wine polyphenol, resveratrol in cultured aortic smooth muscle cells leads to cytoprotection against oxidative and electrophilic stress. Pharmacological Research 2006, 53, 6-15, 10.1016/j.phrs.2005.08.002.
- Rodrigo, R.; Miranda-Merchak, A.; Grau, R. V.; Bachler, J. P.; Vergara, L.; Modulation of (Na,K)-ATPase activity by membrane fatty acid composition: Therapeutic implications in human hypertension.. Clin. Exp. Hypertens. 2014, 36, 17-26, doi:10.3109/10641963.2013.783048..
- Dimitris Tousoulis; George Bouras; Charalambos Antoniades; Kyriakoula Marinou; Antigoni Miliou; Nikos Papageorgiou; George Chatzis; Costas Tentolouris; Costas Tsioufis; Christodoulos Stefanadis; et al. The activation of endothelin-1 pathway during methionine-induced homocysteinemia mediates endothelial dysfunction in hypertensive individuals. Journal of Hypertension 2010, 28, 925-930, 10.1097/hjh.0b013e32833778b2.
- Mathias D G Van Den Eynde; Johanna M Geleijnse; Jean L J M Scheijen; Nordin M J Hanssen; James I Dower; Lydia A Afman; Coen D A Stehouwer; Peter C H Hollman; Casper G Schalkwijk; Quercetin, but Not Epicatechin, Decreases Plasma Concentrations of Methylglyoxal in Adults in a Randomized, Double-Blind, Placebo-Controlled, Crossover Trial with Pure Flavonoids. The Journal of Nutrition 2018, 148, 1911-1916, 10.1093/jn/nxy236.
- Maria Saarenhovi; Pia Salo; Mika Scheinin; Jussi Lehto; Zsófia Lovró; Kirsti Tiihonen; Markus J. Lehtinen; Jouni Junnila; Oliver Hasselwander; Anneli Tarpila; et al.Olli T. Raitakari The effect of an apple polyphenol extract rich in epicatechin and flavan-3-ol oligomers on brachial artery flow-mediated vasodilatory function in volunteers with elevated blood pressure. Nutrition Journal 2017, 16, 73-73, 10.1186/s12937-017-0291-0.
- Joanna M. Young; Christopher M. Florkowski; Sarah L. Molyneux; Roberta G. McEwan; Christopher M. Frampton; M. Gary Nicholls; Russell S. Scott; Peter M. George; A Randomized, Double-Blind, Placebo-Controlled Crossover Study of Coenzyme Q10 Therapy in Hypertensive Patients With the Metabolic Syndrome. American Journal of Hypertension 2012, 25, 261-270, 10.1038/ajh.2011.209.
- Ali Abd El-Aal; Eman A. Abd El-Ghffar; Asmaa Abu Ghali; Mohammed R. Zughbur; Mahmoud M. Sirdah; The effect of vitamin C and/or E supplementations on type 2 diabetic adult males under metformin treatment: A single-blinded randomized controlled clinical trial. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 2018, 12, 483-489, 10.1016/j.dsx.2018.03.013.
- Baumgartner, S.; Mensink, R. P.; Haenen, G. R.; Bast, A.; Binder, C. J.; Bekers, O.; Husche, C.; Lütjohann, D.; Plat, J.; The effects of Vitamin E or lipoic acid supplementation on oxyphytosterols in subjects with elevated oxidative stress: A randomized trial. Sci. Rep 2017, 7, 1-9, doi:10.1038/s41598-017-15615-y..
- Maryam Rafraf; Behnaz Bazyun; Mohammad Ali Sarabchian; Abdolrasoul Safaeiyan; Bahram Pourghassem Gargari; Vitamin E Improves Serum Paraoxonase-1 Activity and Some Metabolic Factors in Patients with Type 2 Diabetes: No Effects on Nitrite/Nitrate Levels. Journal of the American College of Nutrition 2016, 35, 521-528, 10.1080/07315724.2015.1116896.
- Rachel-Marie Cazeau; Hong Huang; John Anthony Bauer; Robert P. Hoffman; Effect of Vitamins C and E on Endothelial Function in Type 1 Diabetes Mellitus. Journal of Diabetes Research 2015, 2016, 1-5, 10.1155/2016/3271293.
- Baumgartner, S.; Mensink, R. P.; Haenen, G. R.; Bast, A.; Binder, C. J.; Bekers, O.; Husche, C.; Lütjohann, D.; Plat, J.; The effects of Vitamin E or lipoic acid supplementation on oxyphytosterols in subjects with elevated oxidative stress: A randomized trial. Sci. Rep 2017, 7, 1-9, doi:10.1038/s41598-017-15615-y.
- A. Sattarinezhad; J. Roozbeh; B. Shirazi Yeganeh; G.R. Omrani; M. Shams; Resveratrol reduces albuminuria in diabetic nephropathy: A randomized double-blind placebo-controlled clinical trial. Diabetes & Metabolism 2019, 45, 53-59, 10.1016/j.diabet.2018.05.010.
- Shadisadat Seyyedebrahimi; Hadi Khodabandehloo; Ensieh Nasli Esfahani; Reza Meshkani; The effects of resveratrol on markers of oxidative stress in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled clinical trial. Acta Diabetologica 2018, 55, 341-353, 10.1007/s00592-017-1098-3.
- Simona Bo; Gabriele Togliatto; Roberto Gambino; Valentina Ponzo; Giusy Lombardo; Rosalba Rosato; Maurizio Cassader; Maria Felice Brizzi; Impact of sirtuin-1 expression on H3K56 acetylation and oxidative stress: a double-blind randomized controlled trial with resveratrol supplementation. Acta Diabetologica Latina 2018, 55, 331-340, 10.1007/s00592-017-1097-4.
- Haruki Imamura; Takashi Yamaguchi; Daiji Nagayama; Atsuhito Saiki; Kohji Shirai; Ichiro Tatsuno; Resveratrol Ameliorates Arterial Stiffness Assessed by Cardio-Ankle Vascular Index in Patients With Type 2 Diabetes Mellitus. International Heart Journal 2017, 58, 577-583, 10.1536/ihj.16-373.
- Pál Brasnyó; Gergő A. Molnár; Márton Mohás; Lajos Markó; Boglárka Laczy; Judit Cseh; Esztella Mikolás; István András Szijártó; Ákos Mérei; Richárd Halmai; et al.László G. MészárosBalázs SümegiIstván Wittmann Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. British Journal of Nutrition 2011, 106, 383-389, 10.1017/s0007114511000316.
- Giuseppe DeRosa; Angela D'angelo; Davide Romano; Pamela Maffioli; A Clinical Trial about a Food Supplement Containing α-Lipoic Acid on Oxidative Stress Markers in Type 2 Diabetic Patients.. International Journal of Molecular Sciences 2016, 17, 1802, 10.3390/ijms17111802.
- Supatra Porasuphatana; Suthi Suddee; Atinuch Nartnampong; Julraht Konsil; Busakorn Harnwong; Adichai Santaweesuk; Glycemic and oxidative status of patients with type 2 diabetes mellitus following oral administration of alpha-lipoic acid: a randomized double-blinded placebo-controlled study.. Asia Pacific Journal of Clinical Nutrition 2012, 21, 12-21.
- Mariana Magalhães Carrepeiro; Marcelo Macedo Rogero; Marcelo Chiara Bertolami; Patrícia Borges Botelho; Natalia Castro; Inar Alves Castro; Effect of n-3 fatty acids and statins on oxidative stress in statin-treated hypercholestorelemic and normocholesterolemic women. Atherosclerosis 2011, 217, 171-178, 10.1016/j.atherosclerosis.2010.12.013.
- Keren Doenyas-Barak; Sylvia Berman; Ramzia Abu-Hamad; Ahuva Golik; Naomi Rahimi-Levene; Shai Efrati; N-3 fatty acid supplementation to routine statin treatment inhibits platelet function, decreases patients’ daytime blood pressure, and improves inflammatory status. European Journal of Clinical Pharmacology 2012, 68, 1139-1146, 10.1007/s00228-012-1235-4.
- C. Apostolidou; K. Adamopoulos; S. Iliadis; C. Kourtidou-Papadeli; Alterations of antioxidant status in asymptomatic hypercholesterolemic individuals after resveratrol intake. International Journal of Food Sciences and Nutrition 2016, 67, 541-552, 10.3109/09637486.2016.1174192.
- Stefanie B. Murer; Isabelle Aeberli; Christian P. Braegger; Matthias Gittermann; Martin Hersberger; Scott W. Leonard; Alan W. Taylor; Maret G. Traber; Michael B. Zimmermann; Antioxidant Supplements Reduced Oxidative Stress and Stabilized Liver Function Tests but Did Not Reduce Inflammation in a Randomized Controlled Trial in Obese Children and Adolescents. The Journal of Nutrition 2013, 144, 193-201, 10.3945/jn.113.185561.
- G.M.S. Batista; H.N.M. Rocha; A.S. Storch; V.P. Garcia; G.F. Teixeira; J. Mentzinger; E.A.C. Gomes; L.L. Velasco; A.C.L. Nobrega; N.G. Rocha; et al. Ascorbic acid inhibits vascular remodeling induced by mental stress in overweight/obese men. Life Sciences 2020, 250, 117554, 10.1016/j.lfs.2020.117554.
- Mehrangiz Ebrahimi-Mameghani; Haleh Jamali; Reza Mahdavi; Farzad Kakaei; Rana Abedi; Bita Kabir-Mamdooh; Conjugated linoleic acid improves glycemic response, lipid profile, and oxidative stress in obese patients with non-alcoholic fatty liver disease: a randomized controlled clinical trial. Croatian Medical Journal 2016, 57, 331-341, 10.3325/cmj.2016.57.331.
- Jasper Most; Ines Warnke; Mark V. Boekschoten; Johan W. E. Jocken; Philip De Groot; Angelika Friedel; Igor Bendik; Gijs H. Goossens; Ellen E. Blaak; The effects of polyphenol supplementation on adipose tissue morphology and gene expression in overweight and obese humans. Adipocyte 2018, 7, 190-196, 10.1080/21623945.2018.1469942.
- R.H.X. Wong; P.R.C. Howe; J.D. Buckley; A.M. Coates; I. Kunz; N.M. Berry; Acute resveratrol supplementation improves flow-mediated dilatation in overweight/obese individuals with mildly elevated blood pressure. Nutrition, Metabolism and Cardiovascular Diseases 2011, 21, 851-856, 10.1016/j.numecd.2010.03.003.
- Eunice Mah; Ruisong Pei; Yi Guo; Kevin D. Ballard; Tyler Barker; Victoria E. Rogers; Beth A. Parker; Alan W. Taylor; Maret G. Traber; Jeff S. Volek; et al.Richard S. Bruno γ-Tocopherol-rich supplementation additively improves vascular endothelial function during smoking cessation. Free Radical Biology and Medicine 2013, 65, 1291-1299, 10.1016/j.freeradbiomed.2013.09.016.
- Mah, E.; Pei, R.; Guo, Y.; Masterjohn, C.; Ballard, K. D.; Taylor, B. A.; Taylor, A. W.; Traber, M. G.; Volek, J. S.; Bruno, R. S.; et al. Greater γ-tocopherol status during acute smoking abstinence with nicotine replacement therapy improved vascular endothelial function by decreasing 8-iso-15(S)-prostaglandin F2α.. Exp. Biol. Med. 2015, 240, 527-533, doi:10.1177/1535370214556948..
- Kristin A. Guertin; Rachael K. Grant; Kathryn B. Arnold; Lindsay Burwell; Joann Hartline; Phyllis J. Goodman; Lori M. Minasian; Scott M. Lippman; Eric Klein; Patricia A. Cassano; et al. Effect of long-term vitamin E and selenium supplementation on urine F2-isoprostanes, a biomarker of oxidative stress. Free Radical Biology and Medicine 2016, 95, 349-356, 10.1016/j.freeradbiomed.2016.03.010.
- Kiana Sadeghi-Ardekani; Mahmonir Haghighi; Rasoul Zarrin; Effects of omega-3 fatty acid supplementation on cigarette craving and oxidative stress index in heavy-smoker males: A double-blind, randomized, placebo-controlled clinical trial. Journal of Psychopharmacology 2018, 32, 995–1002, 10.1177/0269881118788806.
- S. Bo; G. Ciccone; A. Castiglione; R. Gambino; F. De Michieli; P. Villois; M. Durazzo; P. Cavallo-Perin; M. Cassader; Anti-Inflammatory and Antioxidant Effects of Resveratrol in Healthy Smokers A Randomized, Double-Blind, Placebo-Controlled, Cross-Over Trial. Current Medicinal Chemistry 2013, 20, 1323-1331, 10.2174/0929867311320100009.