Fugitive dust is a serious threat to unpaved road users from a safety and health point of view. Dust suppressing materials or dust suppressants are often employed to lower the fugitive dust. Currently, many dust suppressants are commercially available and are being developed for various applications.
- Fugitive Dust
- dust suppressants
1. Introduction
In the United States, unpaved or gravel roads constitute about 33.1% of the complete road network [1]. A significant portion of these unpaved roads serve as a connection between rural farming communities and urban areas, and the rest of them facilitate pathways to forests, mining fields, and timber hauls [2]. On unpaved roads, fugitive dust emanates from the mechanical interaction between the moving vehicles and the crushed aggregates [3]. Fugitive dust primarily comprises soil minerals (e.g., oxides of silicon, aluminum, calcium, and iron) with particulate material sizes lower than 10 μm (PM
) [4]. According to the National Transportation Statistics (NTS) report [1], approximately 18.5 million short tons of PM
and 5.34 million short tons of PM
particulates (size lower than 2.5 μm) are entrained into the air annually. About 35% of this particulate material comes from unpaved roads [5]. From the health, economic, and safety points of view, the generation of fugitive dust poses a serious threat to road users and people living in the vicinity of unpaved roads.
The presence of PM
and PM
in the fugitive dust is found to significantly impact the health of the public, livestock, vegetation, and aquatic life in the premises of unpaved roads by promoting the transport of allergens, spores, and microorganisms [6][7]. While some researchers reported a positive association between PM
particulates and the cardiovascular and respiratory complications [8][9][10][11][12][13][14], other researchers reported a positive association between PM
and higher rates of hospitalization due to ischemic heart disease and carcinoma [15][16][17]. A more detailed literature review on various chronic diseases resulting from the fugitive dust can be found elsewhere [18][19][20][21]. In a recent data analysis carried out by Wu et al. [22] using data from the United States, a positive association between the long-term exposure to PM
and the increased risk of COVID-19 (Coronavirus disease 2019) was reported, i.e., an increase of only 1 μg/m
in PM
is associated with an 8% increase in the COVID-19 death rate in the United States. In the case of children and elderly people, the finer dust particulates were noticed to aggravate heart and lung diseases such as bronchitis, pneumonitis, wheezing, cardiac artery disease, and cardiac arrhythmias, which can increase the risk of death [23].
When spread in air in higher concentrations, fugitive dust not only adversely impacts the air quality but also obscures the road visibility, leading to the increased risk of accidents, fatalities, and disruption of smooth flow of traffic [4][24][25]. The rate of fatalities on unpaved rural roads in the United States was reported to be more than double when compared to paved urban roads, i.e., for 100 million vehicle miles traveled, the rate of fatalities on unpaved rural roads is 1.8, and the rate of fatalities on paved rural roads is 0.7 [1][26]. The probability of wind-related accidents was determined to contribute to low visibilities, indicating fugitive dust as one of the possible reasons for this increased accident rate on unpaved roads [6]. Examples of some accidents that occurred in the past due to fugitive dust include a chain of vehicle crashes on I-39 Wisconsin [27]; accidents on Interstate 5 in Coalinga, California; a fatal ATV rollover crash in Carlton country, Minnesota [28]; crashes in the intersection of Conejo Avenue and Highway 41, California [29]; crashes on U.S. Highway 87 between Great Falls and Fort Benton [30]; and accidents in Butler County, Missouri [30]. Numerous individual car crashes and mortalities were also recorded in the past on unpaved roads due to the low visibility and dust storms.
Keeping in view the dreadful impacts of fugitive dust on human health and safety, state departments of transportation (DoTs) and local (county/city/rural) agencies often employ maintenance techniques on these unpaved roads, such as paving, blading, speed control, and chemical stabilization to circumvent the entrainment of fugitive dust and to ensure the safety of unpaved road users [24]. Among these techniques, dust suppressants or chemical stabilizers are most widely adopted in practice due to their ease of application and low cost. The commonly employed dust suppressants to control the fugitive dust include water, calcium chloride (CaCl
), magnesium chloride (MgCl
), and other chloride salts. However, the performances of the dust suppressants vary depending on their physical and chemical characteristics, application rates, soil type, wind speed, atmospheric conditions, etc. Presently, there are more than 200 dust-suppressing products available on the market [31].
To the best of our knowledge, a comprehensive review of contemporary research into the dust-suppressing materials and their working mechanisms is not available in the literature. While it is necessary to understand the characteristics and the working mechanism of dust-suppressing materials from the field application perspective, it is also important to be informed about the process involved in synthesizing the material briefly that would be of interest for many practicing environmental, structural, and transportation engineers. The current review paper not only focuses on describing various working mechanisms involved in suppressing dust using dust suppressants but also provides a brief overview of the process involved in their synthesis. The rest of the manuscript is organized as follows. Description of the dust suppression mechanisms is provided in
; a review of various categories of dust suppressants, their synthesis, and advantages are described in
; and the highlights from the review and the recommendations are provided in
.
2. Dust Suppression Mechanisms
The efficiency of dust suppressants is based on one or both of two underlying mechanisms, namely, hygroscopicity and agglomeration. In this section, a brief description of these two mechanisms is provided.
2.1. Hygroscopicity
Hygroscopicity refers to the ability of a solid substance to absorb or adsorb moisture from the surrounding atmosphere [32][33]. Owing to their affinity to water, hygroscopic substances can retain moisture and maintain a dampened, hard, and compact road surface, which subsequently prevents the erosion of fugitive dust. On the basis of the mechanism of water absorption, one can categorize the hygroscopic materials into two classes, namely, chemical and physical hygroscopic materials [34][35][36]. While chemical hygroscopic materials absorb water via a chemical reaction that converts their entire nature (e.g., metal hydrides), the physical hygroscopic materials imbibe water vapor through the following four mechanisms: (i) surface adsorption, (ii) condensation in capillaries (e.g., soft polyurethane sponge), (iii) reversible changes of the crystal structure (e.g., silica gel and anhydrous inorganic salt), and (iv) combination of capillary forces and hydration of functional groups (e.g., hydrogels and superabsorbent polymers). The detailed description of these mechanisms can be found elsewhere [37]. Interestingly, a hygroscopic substance may deliquesce if its critical relative humidity (CRH) is lower than that of the surrounding atmosphere, i.e., the water adsorbed on the surface of the hygroscopic substance starts to solvate molecules to an extent that the complete substance is liquified [32][38][39]. Often, deliquescent substances (e.g., CaCl
, MgCl
, FeCl
etc.) are employed in practice for dust suppression [40]. The mechanism of dust suppression through hygroscopicity involves four stages (see
a), namely, Stage 1: the deliquescent dust suppressant is sprayed or mixed with the dust particles; Stage 2: deliquescent substance starts to absorb moisture, and water molecules start to accumulate around the deliquescent substance; Stage 3: the outer layer of the deliquescent substance gets dissolved in the absorbed water; and Stage 4: most of the deliquescent substance gets dissolved in the absorbed water that also contains the dust particle in the formed solution, thereby capturing the dust.
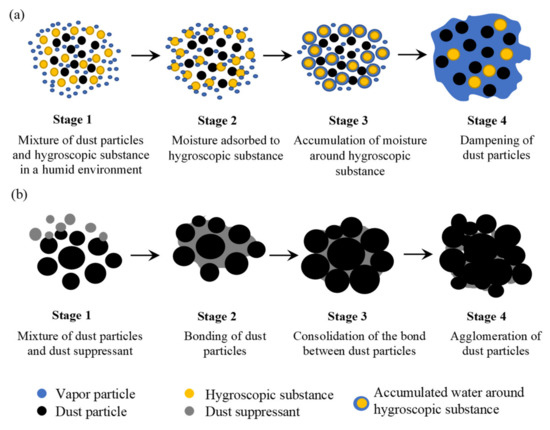
Schematic of dust suppression mechanisms: (
) hygroscopicity and (
) agglomeration.
2.2. Agglomeration
Agglomeration-based dust suppression is obtained when binding or cementing agents are introduced into the dust particles. Agglomeration is referred to as the process of converting small diameter solid particles into larger diameter granules that are composed of smaller particles. The binding or cementing agent introduces adhesive forces among the particles to accumulate a larger number and mass of smaller particles. As the mass of agglomerated particles increase, the constituent dust particles are less prone to become airborne. Examples of agglomeration-based dust suppressants include corn starch hydrogels, guar gum, chitosan, different surfactants, and oil-based substances. Similar to hygroscopicity, the mechanism of dust suppression through agglomeration involves four stages (see
b), namely, Stage 1: the dust suppressant is applied on the top layer of the unpaved road; Stage 2: the dust suppressant starts to form an adhesive bridge among the dust particles; Stage 3: the adhesive bridge starts to solidify between the dust particles; and Stage 4: the lump of the agglomerated dust particles grow in size, thus suppressing fugitive dust. However, agglomeration can take place on the top surface only where the agglomerated particles create a protective layer that resists the particles underneath it to become fugitive.
References
- Bureau of Transportation Statistics. National Transportation Statistics; Bureau of Transportation Statistics: Washington, DC, USA, 2018.
- Addo, J.Q.; Sanders, T.G.; Chenard, M. Road dust suppression: Effect on maintenance stability, safety and the environment; Final Rep. MPC04; The National Academy of Sciences: Washington DC, USA, 2004.
- Zhao, G.; Chen, Y.; Hopke, P.K.; Holsen, T.M.; Dhaniyala, S. Characteristics of traffic-induced fugitive dust from unpaved roads. Aerosol Sci. Technol. 2017, 51, 1324–1331.
- Michigan Department of Environmental Quality. Managing Fugitive Dust: A Guide for Compliance with the Air Regulatory Requirements for Particulate Matter Generation Managing Fugitive Dust; Michigan Department of Environmental Quality: Lansing, MI, USA, 2005.
- Environmental Protection Agency (EPA). NEI Report Dashboard 2014; EPA: Washington, DC, USA, 2014. Available online: (accessed on 13 August 2020).
- Bhattachan, A.; Okin, G.S.; Zhang, J.; Vimal, S.; Lettenmaier, D.P. Characterizing the role of wind and dust in traffic accidents in California. GeoHealth 2019, 3, 328–336.
- Greening, T. Quantifying the Impacts of Vehicle-Generated Dust: A Comprehensive Approach; World Bank: Washington, DC, USA, 2011.
- Bell, M.L.; Dominici, F.; Ebisu, K.; Zeger, S.L.; Samet, J.M. Spatial and temporal variation in PM2.5 chemical composition in the United States for health effects studies. Environ. Health Perspect. 2007, 115, 989–995.
- Bell, M.L.; Ebisu, K.; Leaderer, B.P.; Gent, J.F.; Lee, H.J.; Koutrakis, P.; Wang, Y.; Dominici, F.; Peng, R.D. Associations of PM2.5 constituents and sources with hospital admissions: Analysis of four counties in connecticut and Massachusetts (USA) for persons ≥ 65 years of age. Environ. Health Perspect. 2014, 122, 138–144.
- Bell, M.L.; Belanger, K.; Ebisu, K.; Gent, J.F.; Lee, H.J.; Koutrakis, P.; Leaderer, B.P. Prenatal exposure to fine particulate matter and birth weight. Epidemiology 2010, 21, 884–891.
- Mar, T.F.; Larson, T.V.; Stier, R.A.; Claiborn, C.; Koenig, J.Q. An analysis of the association between respiratory symptoms in subjects with asthma and daily air pollution in Spokane, Washington. Inhal. Toxicol. 2004, 16, 809–815.
- Franklin, M.; Koutrakis, P.; Schwartz, J. The role of particle composition on the association between PM2.5 and mortality. Epidemiology 2008, 19, 680–689.
- Kioumourtzoglou, M.-A.; A Coull, B.; Dominici, F.; Koutrakis, P.; Schwartz, J.; Suh, H. The impact of source contribution uncertainty on the effects of source-specific PM2.5 on hospital admissions: A case study in Boston, MA. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 365–371.
- Ryan, P.H.; Dihle, M.; Griffin, S.; Partridge, C.; Hilbert, T.J.; Taylor, R.; Adjei, S.; Lockey, J.E. Erionite in road gravel associated with interstitial and pleural changes—An occupational hazard in Western United States. J. Occup. Environ. Med. 2011, 53, 892–898.
- Pun, V.C.; Yu, I.T.-S.; Ho, K.-F.; Qiu, H.; Sun, Z.; Tian, L. Differential effects of source-specific particulate matter on emergency hospitalizations for ischemic heart disease in Hong Kong. Environ. Health Perspect. 2014, 122, 391–396.
- Zereini, F.; Alsenz, H.; Wiseman, C.L.; Püttmann, W.; Reimer, E.; Schleyer, R.; Bieber, E.; Wallasch, M. Platinum group elements (Pt, Pd, Rh) in airborne particulate matter in rural vs. urban areas of Germany: Concentrations and spatial patterns of distribution. Sci. Total Environ. 2012, 416, 261–268.
- Ducret-Stich, R.E.; Tsai, M.-Y.; Thimmaiah, D.; Künzli, N.; Hopke, P.K.; Phuleria, H.C. PM10 source apportionment in a Swiss Alpine valley impacted by highway traffic. Environ. Sci. Pollut. Res. 2013, 20, 6496–6508.
- Khan, R.K.; Strand, M.A. Road dust and its effect on human health: A literature review. Epidemiol. Health 2018, 40, e2018013.
- Alves, C.A.; Vicente, E.D.; Vicente, A.M.; Rienda, I.C.; Tomé, M.; Querol, X.; Amato, F.; Rienda, I.C. Loadings, chemical patterns and risks of inhalable road dust particles in an Atlantic city in the north of Portugal. Sci. Total Environ. 2020, 737, 139596.
- Jose, J.; Srimuruganandam, B. Investigation of road dust characteristics and its associated health risks from an urban environment. Environ. Geochem. Health 2020, 42, 2819–2840.
- Chen, S.; Zhang, X.; Lin, J.; Huang, J.; Zhao, D.; Yuan, T.; Huang, K.; Luo, Y.; Jia, Z.; Zang, Z.; et al. Fugitive road dust PM2.5 emissions and their potential health impacts. Environ. Sci. Technol. 2019, 53, 8455–8465.
- Wu, X.; Nethery, R.C.; Sabath, B.M.; Braun, D.; Dominici, F. Exposure to air pollution and COVID-19 mortality in the United States: A nationwide cross-sectional study. medRxiv 2020.
- USDA. Fugitive Dust A Guide to the Control of Windblown Dust on Agricultural Lands in Nevada; USDA: Washington, DC, USA, 2007.
- Succarieh, M. Control of Dust Emissions from Unpaved Roads; Washington, DC, USA, 1992; Available online: (accessed on 21 June 2020).
- Ashley, W.S.; Strader, S.; Dziubla, D.C.; Haberlie, A. Driving blind: Weather-related vision hazards and fatal motor vehicle crashes. Bull. Am. Meteorol. Soc. 2015, 96, 755–778.
- Ecola, L.; Popper, S.; Silberglitt, R.; Fraade-Blanar, L. The road to zero: A vision for achieving zero roadway deaths by 2050. Rand Health Q. 2018, 8, 11.
- Jones, M. Plainfield Dust Storm: Crashes on Wisconsin I-39 Involve 37 Vehicles 2020. Available online: (accessed on 21 June 2020).
- Walsh, P. Dust May Have Been Factor in Fatal ATV Crash near Moose Lake. 2020. Available online: (accessed on 21 June 2020).
- Parmer, J. Coroner Identifies Victims in Fatal Crash on Highway 41; Nexstar Broadcast Inc.: Irving, TX, USA, 2020; Available online: (accessed on 21 June 2020).
- Higgins, G.; Murray, D. Highway 87 between Great Falls and Fort Benton Closed due to High Winds. Great Falls Tribune, 1 February 2020.
- Jones, D. Guideline for the Selection, Specification, Application of Chemical Dust Control and Stabilization Treatments on Unpaved Roads; University of California Pavement Research Center: Davis, CA, USA, 2017.
- Ford, J.L.; Willson, R. Thermal Analysis and Calorimetry of Pharmaceuticals. In Handbook of Thermal Analysis and Calorimetry; Elsevier Science BV: Amsterdam, The Netherlands, 1999; Volume 4, pp. 923–1016.
- Pinterić, M. Building Physics: From Physical Principles to International Standards; Springer International Publishing: Berlin/Heidelberg, Germany, 2017.
- Zohuriaan-Mehr, M.; Kabiri, K. Superabsorbent polymer materials: A review. Iran Polym. J. 2008, 17, 451–477.
- Niazi, S. Handbook of Preformulation: Chemical, Biological, and Botanical Drugs, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2019.
- Mauer, L.J.; Taylor, L.S. Water-solids interactions: Deliquescence. Annu. Rev. Food Sci. Technol. 2010, 1, 41–63.
- Allan, M.C. Characterization of Water-Solid Interactions in Crystalline Ingredients and Development of Deliquescence Measurement Recommendations; Purdue University: West Lafayette, IN, USA, 2014.
- Tereshchenko, A.G. Deliquescence: Hygroscopicity of water-soluble crystalline solids. J. Pharm. Sci. 2015, 104, 3639–3652.
- Yao, W.; Yu, X.; Lee, J.W.; Yuan, X.; Schmidt, S.J. Measuring the deliquescence point of crystalline sucrose as a function of temperature using a new automatic isotherm generator. Int. J. Food Prop. 2011, 14, 882–893.
- Du, L.; Wang, Z.; Li, S.; Song, W.; Lin, W. A comparison of monomeric phenols produced from lignin by fast pyrolysis and hydrothermal conversions. Int. J. Chem. React. Eng. 2013, 11, 135–145.
