Critical limb ischemia (CLI) constitutes the most severe form of peripheral arterial disease (PAD), a prevalent manifestation of atherosclerosis which involves the blockade of major systemic arteries other than those of the cerebral and coronary circulation, more common in legs than in arms. Overall, CLI patients suffer from chronic ischemic rest pain, ulcers, or gangrene, as well as an increased risk of cardiovascular events. CLI has a huge impact on the patients’ quality of life, being associated with an increased risk of amputations (fingers, toes, or extremities) and, moreover, an increase in mortality rates.
Critical limb ischemia (CLI) constitutes the most severe form of peripheral arterial disease (PAD), a prevalent manifestation of atherosclerosis which involves the blockade of major systemic arteries other than those of the cerebral and coronary circulation, more common in legs than in arms. Overall, CLI patients suffer from chronic ischemic rest pain, ulcers, or gangrene, as well as an increased risk of cardiovascular events. CLI has a huge impact on the patients’ quality of life, being associated with an increased risk of amputations (fingers, toes, or extremities) and, moreover, an increase in mortality rates.
Currently, revascularization strategies (bypass grafting, angioplasty) remain the first option for CLI patients, although less than 45% of them are eligible for surgical intervention mainly due to associated comorbidities. Moreover, patients usually require amputation in the short-term. As an alternative to conventional treatments, therapeutic angiogenesis has arisen as a promising treatment for CLI patients, mainly those considered as “no-option”, due to the potential of this strategy to promote revascularization of ischemic tissues. Different approaches including angiogenic gene or cell-based therapies are currently under investigation.
- critical limb ischemia
- neovascularization
- angiogenesis
- arteriogenesis
- cell therapy
- secretomes
1. Critical Limb Ischemia
Critical Limb Ischemia (CLI) constitutes the most severe form of Peripheral Arterial Disease (PAD), a prevalent manifestation of atherosclerosis which involves the blockade of major systemic arteries other than those of the cerebral and coronary circulation
[1]
, more common in legs than in arms
[2]
. PAD affects around 10–15% of adults, being an underestimated and underdiagnosed cardiovascular disease (CVD) due to its asymptomatic initial stages
[3]
. PAD is associated with risk factors such as older age, hypertension, dyslipidemia, or smoking
[4]
, and it is more prevalent in diabetic people due to metabolic alterations such as angiogenesis impairment, inflammatory progression, or endothelial dysfunction
. CLI itself has an annual incidence of 0.35% and an average prevalence of 1.33%, affecting to 500–1000 people per 1 million population in Europe and the United States
[9]
. CLI patients are classified based on clinical criteria and hemodynamic parameters (i.e., pulse volume recordings, ankle and toe pressure values, rest pain, and tissue loss)
currently accepted in international consensus guidelines on PAD and CLI
. Overall, CLI patients suffer from chronic ischemic rest pain, ulcers, or gangrene, as well as an increased risk of cardiovascular events. CLI has a huge impact on the patients’ quality of life, being associated with an increased risk of amputations (fingers, toes, or extremities) and, moreover, an increase in mortality rates
. This debilitating disease causes high dependency on caregivers, requiring permanent local wound treatment, and the chronic use of pain-relieving medications, considerably diminishing patient’s quality of life
[21]
.
Nowadays, the treatment of CLI remains highly variable and, in many situations, suboptimal
[22]
. Initial recommendations for CLI patients to prevent further cardiovascular events include smoking cessation, lipid lowering (statins mainly), antiplatelet therapies, or ACE inhibitors
[16]
. Alternatively, other medical strategies or pharmaceutical agents have been applied for the specific treatment of CLI patients (sympathectomy or spinal cord stimulation, iloprost)
[23]
. Unfortunately, these strategies do not seem to be totally effective in reducing limb-specific events
[16]
, although larger studies/clinical trials are required in order to reach definitive conclusions.
The majority of CLI patients require revascularization interventions like bypass or angioplasty, having observed a significant improvement in the techniques and devices applied (cryoplasty, stent-grafts, drug-eluting balloons or stents, etc.) in the past decades. Nevertheless, the percentage of patients eligible for these strategies is not higher than 45% due to high comorbidity or surgical related issues such as difficult access due to narrow vessels, etc. Furthermore, patients that undergo surgery will usually require amputation at the short term
[24]
. Amputation rates are unacceptably high, typically exceeding 15–20% at 1 year and can vary by the presence of comorbid conditions
[25]
such as diabetes mellitus (DM), which elevates this rate up to 50% in CLI diabetic patients
[26]
. Diabetic patients have higher risk of suffering PAD/CLI and a negative outcome partly related to the abrogation of new vessel formation and remodeling of the pre-existing vasculature under hyperglycemic conditions
[27]
. Unfortunately, the increasing prevalence of PAD together with higher presence of other CLI risk factors (i.e., diabetes) and the rising number of people in advanced age provide little reason to believe that the number of patients suffering this disease will decrease in the near future
[25]
. The poor prognosis of CLI patients as well as their impaired quality of life makes compulsory to find effective and less invasive treatments. Moreover, the desirable treatment should be applicable to all CLI patients, because the actual percentage of ineligible patients is unacceptably high.
As an alternative to conventional treatments, therapeutic angiogenesis has arisen as a promising treatment for CLI patients, mainly those considered as “no-option”, due to the potential of this strategy to promote revascularization of ischemic tissues
. To date, different approaches including angiogenic gene or cell-based therapies are currently under investigation.
We have mainly focused on the use of angiogenic cell therapy for CLI (
Figure 1), from animal/pre-clinical models designed to study CLI and the tools applied to test for revascularization in response to cell therapy, to the angiogenic therapies currently under evaluation in clinical trials. Moreover, recent alternatives derived from stem cell therapies, such as the use of secretomes, exosomes, or even microRNAs, will be described.
), from animal/pre-clinical models designed to study CLI and the tools applied to test for revascularization in response to cell therapy, to the angiogenic therapies currently under evaluation in clinical trials. Moreover, recent alternatives derived from stem cell therapies, such as the use of secretomes, exosomes, or even microRNAs, will be described.
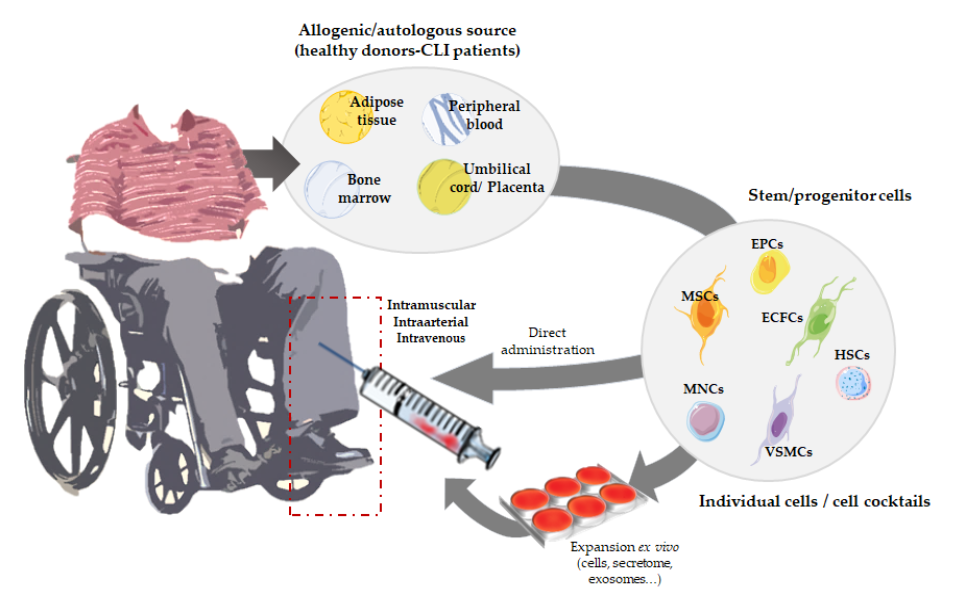
Figure 1. Overview of angiogenic cell therapy for Critical Limb Ischemia (CLI).
2. Angiogenic Cell Therapy
Overview of angiogenic cell therapy for Critical Limb Ischemia (CLI).
2. Angiogenic Cell Therapy
Angiogenic therapy involves the use of angiogenic growth factors (VEGF, HIF-1a, FGF1, HGF, etc.) [33][34], gene transfer techniques using viral or non-viral vectors to transport a gene codifying for a therapeutic protein to the target tissues [35] or, alternatively, the use of angiogenic stem cells. All these strategies aim to improve revascularization by increasing the number/size of blood vessels, promoting blood flow recovery and therefore increasing tissue perfusion in the ischemic extremities [35]. Among them, cell-based therapies seem more efficient compared to protein- or gene-based approaches, not only because of their direct vasculogenic properties, but also due to their paracrine effect. Angiogenic cells can directly participate in the formation of new vessels, while in parallel they also provide endogenous growth factors, promoting vascular growth by paracrine fashion [36][37].
Thus, neovascularization can also be promoted by vasculogenesis, the novo formation of vessels mediated by circulating progenitors or stem cells
[38]
. Vasculogenesis was initially considered as an embryogenic process. However, post-natal vasculogenesis can also take place by incorporation of vascular stem or progenitor cells into vessel structures, allowing the formation of adult blood vessels
[39]
. To date, several strategies based on the use of stem and progenitor cells are being tested (
), to promote vasculogenesis but also angiogenesis and arteriogenesis. The safety and efficacy of cell implantation therapies make of this less invasive treatment a feasible option for CLI patients.
2.1. Cell Therapies Based on Single or Combined Isolated Cells
Mesenchymal stem cells (MSCs) are the most used cells in advanced therapies for CVDs
[40]
. MSCs can be isolated from bone marrow, peripheral blood, or adipose tissues, and from them we can obtain osteoblasts, chondrocytes, adipocytes, neurons, endothelial cells (ECs), skeletal muscle cells, and vascular smooth muscle cells (VSMCs)
[41]
. MSCs are reported to promote angiogenesis because of their capacity to induce ECs proliferation, migration, and tube formation, while decreasing apoptosis and fibrosis
. Furthermore, MSCs support neoangiogenesis, releasing soluble factors that contribute to stimulate angiogenesis
[44]
. These cells are thought to improve hind limb ischemia by secreting cytokines that regulate macrophage differentiation to M2, an anti-inflammatory phenotype
[45]
. Likewise, apart from MSCs, endothelial progenitor cells (EPCs) also represent an important group of cells used in vascular regeneration. In 1997, Asahara et al. demonstrated that CD34+ cells can be isolated from peripheral blood mononuclear cells (PB-MNCs) and differentiated in vitro into ECs, showing the potential use for collateral vessel growth augmentation in ischemic tissues
[46]
. Although CD34 is not a specific marker of a single cell type, it is mostly associated to EPCs. Many researchers have explored the potential of using EPCs in tissue engineering as an angiogenic source for vascular repairing
. In the past years, several isolation and culturing techniques for EPCs have been described. Besides, the controversy regarding the definition of EPC phenotypes remains, with different studies still presenting a variety of results in terms of surface-based EPC markers
. At least, two different sub-populations have been accepted and clearly defined, based on their differentiation status and the capability to form colonies: early EPCs (eEPCs) also named circulating angiogenic cells (CACs) or myeloid angiogenic cells (MACs), with hematopoietic phenotype, and late EPCs or endothelial colony forming cells (ECFCs), with endothelial phenotype
[51]
. EPCs have been thought to derive from hematopoietic stem cells (HSCs), some EPCs could be derived from a niche close to the vasa vasorum in the macro-vascular wall
[52]
. Despite the controversy regarding the nature of these cells, no one denies the potential of EPCs to promote therapeutic angiogenesis and neovascularization of ischemic tissues
. Overall, in response to injury, cytokines and growth factors mobilize EPCs from the bone marrow into the peripheral blood, which will then participate in neovascularization
[53]
. Very recently, we have shown how, first days after administration of CACs to ischemic CLI mice, these cells migrate into the ischemic tissues, modulating immune cells recruitment and promoting an increase of angiogenesis and arteriogenesis
[49]
. However, the administered cells do not remain in the ischemic tissues over time suggesting that they may promote vasculogenesis in a paracrine form
. Moreover, early EPCs do not seem to differentiate to ECs, with this role being assigned to ECFCs
. Indeed, different studies support that the regenerative properties of eEPCs are mainly due to paracrine effects, while ECFCs present vessel-forming activity in vivo
. Thus, a cell therapy mediated by both cell types, early, and late EPCs, could be a good strategy for CVDs. Yoon et al. evaluated this combined cell therapy, demonstrating a synergistic neovascularization involving several cytokines and matrix metalloproteinases (MMPs)
[57]
. Very recently, our group has also corroborated the potential of CACs to promote angiogenesis of ECFCs in vitro, and such effect was impaired under an atherosclerotic environment
[58]
. In the same way, different cell combinations have been tested. Rossi et al. demonstrated that co-injection of MSCs with ECFCs in a murine model of CLI increased vessel density and foot perfusion in greater ratio than cells individually administrated; corroborating the theory that MSCs support ECFC-mediated angiogenic processes
[59]
. Furthermore, their results indicated that MSCs accelerated muscle recovery via endoglin dependent mechanism. Similarly, the combination of EPCs and smooth muscle progenitor cells (SMPCs) has also been evaluated to treat CLI. This cell mixture improved vascular network formation, with both ECs and smooth muscle cells (SMCs) participating in vessel maturation and stability. Likewise, Foubert et al. demonstrated that co-administration of EPCs and SMPCs activates neovascularization resulting in a more effective therapy than these cells administrated separately
[60]
. Some studies suggest that SMCs may also originate from bone marrow-derived cells as SMPCs have been identified in peripheral blood
[61]
.
2.2. Cell Therapies Based on Cellular Cocktails
As an alternative to the injection of a single cell type or the combination of two previously isolated cells, the administration of cellular cocktails derived from different niches, such as bone marrow, peripheral blood, or adipose tissue, is also a frequent approach to treat CLI. Indeed, the regenerative properties of mononuclear cells (MNCs) derived from either bone marrow or peripheral blood have been largely studied in the last years. Therapies employing bone marrow mononuclear cells (BM-MNCs) constitute a promising alternative for CLI patients to avoid or delay the onset of amputation [62]. BM-MNCs consist of a heterogeneous mix of multipotent stem cells working cooperatively as MSCs, HSCs, EPCs, monocytes, lymphocytes, and pluripotent stem cells [63][64]. We and other researchers have reported the beneficial effects of different combinations of BM-MNCs, representing an effective approach in promoting new vessel formation, perfusion recovery, and CLI reversal [63][44][65][66][67][68][69][70][71][72][73]. In the ischemic tissue, BM-MNCs produce and secrete different cytokines and growth factors [74] and increase neovascularization and collateral vessel formation in limb ischemia [75]. Moreover, Kikuchi-Taura et al. have recently described that transplantation of BM-MNCs into a murine stroke model promoted ECs angiogenesis by gap junction mediated cell–cell interactions, elucidating a new theory of how cell-based therapies work, and suggesting that stem cells supply energy to injured cells [76]. This study suggested that, under hypoxic conditions, transplanted BM-MNCs are capable to transfer small molecules to ECs via gap junction interactions, leading to HIF-1α activation, which induced upregulation of VEGF uptake into ECs and ECs autophagy suppression [76].
Alternatively to BM-MNCs, PB-MNCs are formed by circulating cells with angiogenic potential, thereby several studies involving the administration of these cells to treat CLI have also shown promising results [77][78]. Li et al. made a comparison between CD34+ and CD34- cells in PB-MNCs, concluding that both induce neovascularization, but only CD34+ incorporate into new capillaries [79]. PB-MNCs promote revascularization in ischemic limbs, even more when they are combined with platelet-rich plasma (PRP) [80]. PRP, a source of platelets, cytokines, and growth factors, participates in ECs proliferation and differentiation, interacting with important cell receptors related with angiogenesis [80]. Furthermore, in order to achieve high stem cell concentrations, hematopoietic growth factors are frequently used to induce cell mobilization. For example, prior PB-MNCs harvesting, progenitor cells are usually mobilized injecting granulocyte colony-stimulating factor (G-CSF) [77][78][81][82]. BM-MNCs and PB-MNCs treatments have been compared, and no significant differences have been observed between them [83][84]. Remarkably, without previous mobilization, PB-MNCs show higher concentration of mature cells as red blood cells, platelets, lymphocytes, and monocytes, while BM-MNCs show higher levels of EPCs [85].
The use of adipose tissue-derived stem cells (ASCs) has increased in the last years, due to the easier accessibility, abundance, and less painful collection compared to other sources such as bone marrow [86]. The stromal vascular fraction (SVF) derived from adipose tissue contains heterogeneous cell populations such as mesenchymal progenitor/stem cells, pre-adipocytes, endothelial cells, pericytes, T cells, and M2 macrophages. SVF-derived mesenchymal progenitor/stem cells, usually referred as ASCs themselves, can be easily expanded in vitro and have the potential to differentiate into multiple lineages, including myogenic, osteogenic, neurogenic, and hematopoietic pathways [87][88][89][90][91]. The angiogenic properties of these cells have been correlated with a strong paracrine activity, secreting an important number of angiogenesis-related cytokines [90]. Moreover, the administration of ASCs to CLI mice promotes a significant recovery of blood flow in ASCs treated mice compared to ischemic, non-treated ones [87]. Very recently, Liu J et al. have shown that the regenerative properties of transplanted ASCs might correlate with an immunomodulatory effect promoted by these cells. In presence of ASCs, a higher number of macrophages can be found in the muscle, with increased presence of M2 macrophages [92], and its administration in a murine model of CLI induces an angiogenic process in the ischemic tissue [87]. The clear advantages of using these cells are easy access and isolation. ASCs are highly abundant in adipose tissue, making almost unnecessary culture expansion of these cells. Moreover, adipose tissue harvesting requires a minimally invasive intervention [93]. A pilot study using adipose-derived regenerative cells (ADRCs) in CLI patients has been recently published [94].
Finally, other cells with multi-differentiation potential such as amniotic fluid derived stem cells (AFSCs) or umbilical cord blood and placenta tissue derived stem/progenitor cells have also been considered. Placenta-derived MSCs stromal-like cells (PLX-PAD) in CLI mice are currently being tested in a Phase III trial (PACE Trial) with atherosclerotic CLI patients (NCT03006770) after promising results in animal assays [95]. Unfortunately, the low availability of these cells together with ethics concerns related to their use, has limited their translation as cell therapies.
References
- Conte, S.M.; Vale, P.R. Peripheral Arterial Disease. Heart Lung Circ. 2018, 27, 427–432.
- Balakumar, P.; Maung, U.K.; Jagadeesh, G. Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharmacol. Res. 2016, 113 Pt A, 600–609.
- van Weel, V.; van Tongeren, R.B.; van Hinsbergh, V.W.; van Bockel, J.H.; Quax, P.H. Vascular growth in ischemic limbs: A review of mechanisms and possible therapeutic stimulation. Ann. Vasc. Surg. 2008, 22, 582–597.
- Krishna, S.M.; Moxon, J.V.; Golledge, J. A review of the pathophysiology and potential biomarkers for peripheral artery disease. Int. J. Mol. Sci. 2015, 16, 11294–11322.
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070.
- Hao, C.; Shintani, S.; Shimizu, Y.; Kondo, K.; Ishii, M.; Wu, H.; Murohara, T. Therapeutic angiogenesis by autologous adipose-derived regenerative cells: Comparison with bone marrow mononuclear cells. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H869–H879.
- Jude, E.B.; Oyibo, S.O.; Chalmers, N.; Boulton, A.J. Peripheral arterial disease in diabetic and nondiabetic patients: A comparison of severity and outcome. Diabetes Care 2001, 24, 1433–1437.
- Pickup, J.C.; Chusney, G.D.; Thomas, S.M.; Burt, D. Plasma interleukin-6, tumour necrosis factor alpha and blood cytokine production in type 2 diabetes. Life Sci. 2000, 67, 291–300.
- Nehler, M.R.; Duval, S.; Diao, L.; Annex, B.H.; Hiatt, W.R.; Rogers, K.; Zakharyan, A.; Hirsch, A.T. Epidemiology of peripheral arterial disease and critical limb ischemia in an insured national population. J. Vasc. Surg. 2014, 60, 686–695 e2.
- Suggested standards for reports dealing with lower extremity ischemia. Prepared by the Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery/North American Chapter, International Society for Cardiovascular Surgery. J. Vasc. Surg. 1986, 4, 80–94.
- Fontaine, R.; Kim, M.; Kieny, R. [Surgical treatment of peripheral circulation disorders]. Helv. Chir. Acta. 1954, 21, 499–533.
- Rutherford, R.B.; Baker, J.D.; Ernst, C.; Johnston, K.W.; Porter, J.M.; Ahn, S.; Jones, D.N. Recommended standards for reports dealing with lower extremity ischemia: Revised version. J. Vasc. Surg. 1997, 26, 517–538.
- Becker, F.; Robert-Ebadi, H.; Ricco, J.B.; Setacci, C.; Cao, P.; de Donato, G.; Eckstein, H.H.; De Rango, P.; Diehm, N.; Schmidli, J.; et al. Chapter I: Definitions, epidemiology, clinical presentation and prognosis. Eur. J. Vasc. Endovasc. Surg. 2011, 42 (Suppl. 2), S4–S12.
- Dormandy, J.A.; Rutherford, R.B. Management of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic Inter-Society Consensus (TASC). J. Vasc. Surg. 2000, 31 Pt 2, S1–S296.
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.; Group, T.I.W. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45 (Suppl. S), S5–S67.
- Teraa, M.; Conte, M.S.; Moll, F.L.; Verhaar, M.C. Critical Limb Ischemia: Current Trends and Future Directions. J. Am. Heart Assoc. 2016, 5, e002938.
- Conte, M.S.; Pomposelli, F.B. Society for Vascular Surgery Practice guidelines for atherosclerotic occlusive disease of the lower extremities management of asymptomatic disease and claudication. Introduction. J. Vasc. Surg. 2015, 61 (Suppl. 3), 1S.
- Hirsch, A.T.; Haskal, Z.J.; Hertzer, N.R.; Bakal, C.W.; Creager, M.A.; Halperin, J.L.; Hiratzka, L.F.; Murphy, W.R.; Olin, J.W.; Puschett, J.B.; et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): A collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006, 113, e463–e654.
- Simpson, E.L.; Kearns, B.; Stevenson, M.D.; Cantrell, A.J.; Littlewood, C.; Michaels, J.A. Enhancements to angioplasty for peripheral arterial occlusive disease: Systematic review, cost-effectiveness assessment and expected value of information analysis. Health Technol. Assess. 2014, 18, 1–252.
- Walter, D.H.; Krankenberg, H.; Balzer, J.O.; Kalka, C.; Baumgartner, I.; Schluter, M.; Tonn, T.; Seeger, F.; Dimmeler, S.; Lindhoff-Last, E.; et al. Intraarterial administration of bone marrow mononuclear cells in patients with critical limb ischemia: A randomized-start, placebo-controlled pilot trial (PROVASA). Circ. Cardiovasc. Interv. 2011, 4, 26–37.
- Lawall, H.; Zemmrich, C.; Bramlage, P.; Amann, B. Health related quality of life in patients with critical limb ischemia. Vasa 2012, 41, 78–88.
- Patel, R.S. Team Approach to Critical Limb Ischemia Care and Research. Tech. Vasc Interv. Radiol. 2016, 19, 101–103.
- Setacci, C.; de Donato, G.; Teraa, M.; Moll, F.L.; Ricco, J.B.; Becker, F.; Robert-Ebadi, H.; Cao, P.; Eckstein, H.H.; De Rango, P.; et al. Chapter IV: Treatment of critical limb ischaemia. Eur. J. Vasc. Endovasc. Surg. 2011, 42 (Suppl. 2), S43–S59.
- Lichtenberg, M.; Schreve, M.A.; Ferraresi, R.; van den Heuvel, D.A.F.; Unlu, C.; Cabane, V.; Kum, S. Surgical and endovascular venous arterialization for treatment of critical limb ischaemia. Vasa 2018, 47, 17–22.
- Duff, S.; Mafilios, M.S.; Bhounsule, P.; Hasegawa, J.T. The burden of critical limb ischemia: A review of recent literature. Vasc Health Risk Manag. 2019, 15, 187–208.
- Spreen, M.I.; Gremmels, H.; Teraa, M.; Sprengers, R.W.; Verhaar, M.C.; Statius van Eps, R.G.; de Vries, J.P.; Mali, W.P.; van Overhagen, H.; Padi; et al. Diabetes Is Associated with Decreased Limb Survival in Patients With Critical Limb Ischemia: Pooled Data From Two Randomized Controlled Trials. Diabetes Care 2016, 39, 2058–2064.
- Howangyin, K.Y.; Silvestre, J.S. Diabetes mellitus and ischemic diseases: Molecular mechanisms of vascular repair dysfunction. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1126–1135.
- Ouma, G.O.; Zafrir, B.; Mohler, E.R., 3rd; Flugelman, M.Y. Therapeutic angiogenesis in critical limb ischemia. Angiology 2013, 64, 466–480.
- Belch, J.; Hiatt, W.R.; Baumgartner, I.; Driver, I.V.; Nikol, S.; Norgren, L.; Van Belle, E.; TAMRIS Committees and Investigators. Effect of fibroblast growth factor NV1FGF on amputation and death: A randomised placebo-controlled trial of gene therapy in critical limb ischaemia. Lancet 2011, 377, 1929–1937.
- Matoba, S.; Tatsumi, T.; Murohara, T.; Imaizumi, T.; Katsuda, Y.; Ito, M.; Saito, Y.; Uemura, S.; Suzuki, H.; Fukumoto, S.; et al. Long-term clinical outcome after intramuscular implantation of bone marrow mononuclear cells (Therapeutic Angiogenesis by Cell Transplantation [TACT] trial) in patients with chronic limb ischemia. Am. Heart J. 2008, 156, 1010–1018.
- Powell, R.J.; Goodney, P.; Mendelsohn, F.O.; Moen, E.K.; Annex, B.H.; Investigators, H.G.F.T. Safety and efficacy of patient specific intramuscular injection of HGF plasmid gene therapy on limb perfusion and wound healing in patients with ischemic lower extremity ulceration: Results of the HGF-0205 trial. J. Vasc. Surg. 2010, 52, 1525–1530.
- van Royen, N.; Schirmer, S.H.; Atasever, B.; Behrens, C.Y.; Ubbink, D.; Buschmann, E.E.; Voskuil, M.; Bot, P.; Hoefer, I.; Schlingemann, R.O.; et al. START Trial: A pilot study on STimulation of ARTeriogenesis using subcutaneous application of granulocyte-macrophage colony-stimulating factor as a new treatment for peripheral vascular disease. Circulation 2005, 112, 1040–1046.
- Ko, S.H.; Bandyk, D.F. Therapeutic angiogenesis for critical limb ischemia. Semin. Vasc. Surg. 2014, 27, 23–31.
- Powell, R.J.; Simons, M.; Mendelsohn, F.O.; Daniel, G.; Henry, T.D.; Koga, M.; Morishita, R.; Annex, B.H. Results of a double-blind, placebo-controlled study to assess the safety of intramuscular injection of hepatocyte growth factor plasmid to improve limb perfusion in patients with critical limb ischemia. Circulation 2008, 118, 58–65.
- Yla-Herttuala, S.; Alitalo, K. Gene transfer as a tool to induce therapeutic vascular growth. Nat. Med. 2003, 9, 694–701.
- Menasche, P. Cell therapy for peripheral arterial disease. Curr. Opin. Mol. Ther. 2010, 12, 538–545.
- Schmidt, C.A.; Amorese, A.J.; Ryan, T.E.; Goldberg, E.J.; Tarpey, M.D.; Green, T.D.; Karnekar, R.R.; Yamaguchi, D.J.; Spangenburg, E.E.; McClung, J.M. Strain-Dependent Variation in Acute Ischemic Muscle Injury. Am. J. Pathol. 2018, 188, 1246–1262.
- Cooke, J.P.; Meng, S. Vascular Regeneration in Peripheral Artery Disease. Arterioscler Thromb. Vasc. Biol. 2020, 40, 1627–1634.
- Asahara, T.; Kawamoto, A. Endothelial progenitor cells for postnatal vasculogenesis. Am. J. Physiol. Cell Physiol. 2004, 287, C572–C579.
- Soria-Juan, B.; Escacena, N.; Capilla-Gonzalez, V.; Aguilera, Y.; Llanos, L.; Tejedo, J.R.; Bedoya, F.J.; Juan, V.; De la Cuesta, A.; Ruiz-Salmeron, R.; et al. Cost-Effective, Safe, and Personalized Cell Therapy for Critical Limb Ischemia in Type 2 Diabetes Mellitus. Front Immunol. 2019, 10, 1151.
- Kim, Y.; Kim, H.; Cho, H.; Bae, Y.; Suh, K.; Jung, J. Direct comparison of human mesenchymal stem cells derived from adipose tissues and bone marrow in mediating neovascularization in response to vascular ischemia. Cell Physiol. Biochem. 2007, 20, 867–876.Wang, Z.; Zheng, L.; Lian, C.; Qi, Y.; Li, W.; Wang, S. Human Umbilical Cord-Derived Mesenchymal Stem Cells Relieve Hind Limb Ischemia by Promoting Angiogenesis in Mice. Stem. Cells Dev. 2019, 28, 1384–1397.
- Wang, Z.; Zheng, L.; Lian, C.; Qi, Y.; Li, W.; Wang, S. Human Umbilical Cord-Derived Mesenchymal Stem Cells Relieve Hind Limb Ischemia by Promoting Angiogenesis in Mice. Stem. Cells Dev. 2019, 28, 1384–1397.Mathew, S.A.; Naik, C.; Cahill, P.A.; Bhonde, R.R. Placental mesenchymal stromal cells as an alternative tool for therapeutic angiogenesis. Cell Mol. Life Sci. 2020, 77, 253–265.
- Mathew, S.A.; Naik, C.; Cahill, P.A.; Bhonde, R.R. Placental mesenchymal stromal cells as an alternative tool for therapeutic angiogenesis. Cell Mol. Life Sci. 2020, 77, 253–265.Cobellis, G.; Maione, C.; Botti, C.; Coppola, A.; Silvestroni, A.; Lillo, S.; Schiavone, V.; Molinari, A.M.; Sica, V. Beneficial effects of VEGF secreted from stromal cells in supporting endothelial cell functions: Therapeutic implications for critical limb ischemia. Cell Transplant. 2010, 19, 1425–1437.
- Cobellis, G.; Maione, C.; Botti, C.; Coppola, A.; Silvestroni, A.; Lillo, S.; Schiavone, V.; Molinari, A.M.; Sica, V. Beneficial effects of VEGF secreted from stromal cells in supporting endothelial cell functions: Therapeutic implications for critical limb ischemia. Cell Transplant. 2010, 19, 1425–1437.Song, Y.; Zhang, T.J.; Li, Y.; Gao, Y. Mesenchymal Stem Cells Decrease M1/M2 Ratio and Alleviate Inflammation to Improve Limb Ischemia in Mice. Med. Sci. Monit. 2020, 26, e923287.
- Song, Y.; Zhang, T.J.; Li, Y.; Gao, Y. Mesenchymal Stem Cells Decrease M1/M2 Ratio and Alleviate Inflammation to Improve Limb Ischemia in Mice. Med. Sci. Monit. 2020, 26, e923287.Asahara, T.; Murohara, T.; Sullivan, A.; Silver, M.; van der Zee, R.; Li, T.; Witzenbichler, B.; Schatteman, G.; Isner, J.M. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997, 275, 964–967.
- Asahara, T.; Murohara, T.; Sullivan, A.; Silver, M.; van der Zee, R.; Li, T.; Witzenbichler, B.; Schatteman, G.; Isner, J.M. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997, 275, 964–967.Edwards, N.; Langford-Smith, A.W.W.; Wilkinson, F.L.; Alexander, M.Y. Endothelial Progenitor Cells: New Targets for Therapeutics for Inflammatory Conditions with High Cardiovascular Risk. Front. Med. 2018, 5, 200.
- Patel, J.; Donovan, P.; Khosrotehrani, K. Concise Review: Functional Definition of Endothelial Progenitor Cells: A Molecular Perspective. Stem Cells Transl. Med. 2016, 5, 1302–1306.Beltran-Camacho, L.; Jimenez-Palomares, M.; Rojas-Torres, M.; Sanchez-Gomar, I.; Rosal-Vela, A.; Eslava-Alcon, S.; Perez-Segura, M.C.; Serrano, A.; Antequera-Gonzalez, B.; Alonso-Pinero, J.A.; et al. Identification of the initial molecular changes in response to circulating angiogenic cells-mediated therapy in critical limb ischemia. Stem. Cell. Res. Ther. 2020, 11, 106.
- Edwards, N.; Langford-Smith, A.W.W.; Wilkinson, F.L.; Alexander, M.Y. Endothelial Progenitor Cells: New Targets for Therapeutics for Inflammatory Conditions with High Cardiovascular Risk. Front. Med. 2018, 5, 200.Chopra, H.H.M.K.; Kwong, D.L.; Zhang, C.F.; Pow, E.H.N. Insights into Endothelial Progenitor Cells: Origin, Classification, Potentials, and prospects. Stem. Cell Int. 2018, 2018, 24.
- Beltran-Camacho, L.; Jimenez-Palomares, M.; Rojas-Torres, M.; Sanchez-Gomar, I.; Rosal-Vela, A.; Eslava-Alcon, S.; Perez-Segura, M.C.; Serrano, A.; Antequera-Gonzalez, B.; Alonso-Pinero, J.A.; et al. Identification of the initial molecular changes in response to circulating angiogenic cells-mediated therapy in critical limb ischemia. Stem. Cell. Res. Ther. 2020, 11, 106.Medina, R.J.; Barber, C.L.; Sabatier, F.; Dignat-George, F.; Melero-Martin, J.M.; Khosrotehrani, K.; Ohneda, O.; Randi, A.M.; Chan, J.K.Y.; Yamaguchi, T.; et al. Endothelial Progenitors: A Consensus Statement on Nomenclature. Stem. Cells Transl. Med. 2017, 6, 1316–1320.
- Chopra, H.H.M.K.; Kwong, D.L.; Zhang, C.F.; Pow, E.H.N. Insights into Endothelial Progenitor Cells: Origin, Classification, Potentials, and prospects. Stem. Cell Int. 2018, 2018, 24.Stitt, A.W.; O’Neill, C.L.; O’Doherty, M.T.; Archer, D.B.; Gardiner, T.A.; Medina, R.J. Vascular stem cells and ischaemic retinopathies. Prog. Retin. Eye. Res. 2011, 30, 149–166.
- Medina, R.J.; Barber, C.L.; Sabatier, F.; Dignat-George, F.; Melero-Martin, J.M.; Khosrotehrani, K.; Ohneda, O.; Randi, A.M.; Chan, J.K.Y.; Yamaguchi, T.; et al. Endothelial Progenitors: A Consensus Statement on Nomenclature. Stem. Cells Transl. Med. 2017, 6, 1316–1320.Lian, W.; Hu, X.; Pan, L.; Han, S.; Cao, C.; Jia, Z.; Li, M. Human primary CD34(+) cells transplantation for critical limb ischemia. J. Clin. Lab. Anal. 2018, 32, e22569.
- Stitt, A.W.; O’Neill, C.L.; O’Doherty, M.T.; Archer, D.B.; Gardiner, T.A.; Medina, R.J. Vascular stem cells and ischaemic retinopathies. Prog. Retin. Eye. Res. 2011, 30, 149–166.Kalka, C.; Masuda, H.; Takahashi, T.; Kalka-Moll, W.M.; Silver, M.; Kearney, M.; Li, T.; Isner, J.M.; Asahara, T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc. Natl. Acad. Sci. USA 2000, 97, 3422–3427.
- Lian, W.; Hu, X.; Pan, L.; Han, S.; Cao, C.; Jia, Z.; Li, M. Human primary CD34(+) cells transplantation for critical limb ischemia. J. Clin. Lab. Anal. 2018, 32, e22569.Ziegelhoeffer, T.; Fernandez, B.; Kostin, S.; Heil, M.; Voswinckel, R.; Helisch, A.; Schaper, W. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ. Res. 2004, 94, 230–238.
- Kalka, C.; Masuda, H.; Takahashi, T.; Kalka-Moll, W.M.; Silver, M.; Kearney, M.; Li, T.; Isner, J.M.; Asahara, T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc. Natl. Acad. Sci. USA 2000, 97, 3422–3427.Yoder, M.C.; Mead, L.E.; Prater, D.; Krier, T.R.; Mroueh, K.N.; Li, F.; Krasich, R.; Temm, C.J.; Prchal, J.T.; Ingram, D.A. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 2007, 109, 1801–1809.
- Ziegelhoeffer, T.; Fernandez, B.; Kostin, S.; Heil, M.; Voswinckel, R.; Helisch, A.; Schaper, W. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ. Res. 2004, 94, 230–238.Yoon, C.H.; Hur, J.; Park, K.W.; Kim, J.H.; Lee, C.S.; Oh, I.Y.; Kim, T.Y.; Cho, H.J.; Kang, H.J.; Chae, I.H.; et al. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: The role of angiogenic cytokines and matrix metalloproteinases. Circulation 2005, 112, 1618–1627.
- Yoder, M.C.; Mead, L.E.; Prater, D.; Krier, T.R.; Mroueh, K.N.; Li, F.; Krasich, R.; Temm, C.J.; Prchal, J.T.; Ingram, D.A. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 2007, 109, 1801–1809.Eslava-Alcon, S.; Extremera-Garcia, M.J.; Sanchez-Gomar, I.; Beltran-Camacho, L.; Rosal-Vela, A.; Munoz, J.; Ibarz, N.; Alonso-Pinero, J.A.; Rojas-Torres, M.; Jimenez-Palomares, M.; et al. Atherosclerotic Pre-Conditioning Affects the Paracrine Role of Circulating Angiogenic Cells Ex-Vivo. Int. J. Mol. Sci. 2020, 21, 5256.
- Yoon, C.H.; Hur, J.; Park, K.W.; Kim, J.H.; Lee, C.S.; Oh, I.Y.; Kim, T.Y.; Cho, H.J.; Kang, H.J.; Chae, I.H.; et al. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: The role of angiogenic cytokines and matrix metalloproteinases. Circulation 2005, 112, 1618–1627.Rossi, E.; Smadja, D.; Goyard, C.; Cras, A.; Dizier, B.; Bacha, N.; Lokajczyk, A.; Guerin, C.L.; Gendron, N.; Planquette, B.; et al. Co-injection of mesenchymal stem cells with endothelial progenitor cells accelerates muscle recovery in hind limb ischemia through an endoglin-dependent mechanism. Thromb. Haemost. 2017, 117, 1908–1918.
- Eslava-Alcon, S.; Extremera-Garcia, M.J.; Sanchez-Gomar, I.; Beltran-Camacho, L.; Rosal-Vela, A.; Munoz, J.; Ibarz, N.; Alonso-Pinero, J.A.; Rojas-Torres, M.; Jimenez-Palomares, M.; et al. Atherosclerotic Pre-Conditioning Affects the Paracrine Role of Circulating Angiogenic Cells Ex-Vivo. Int. J. Mol. Sci. 2020, 21, 5256.Foubert, P.; Matrone, G.; Souttou, B.; Lere-Dean, C.; Barateau, V.; Plouet, J.; Le Ricousse-Roussanne, S.; Levy, B.I.; Silvestre, J.S.; Tobelem, G. Coadministration of endothelial and smooth muscle progenitor cells enhances the efficiency of proangiogenic cell-based therapy. Circ. Res. 2008, 103, 751–760.
- Rossi, E.; Smadja, D.; Goyard, C.; Cras, A.; Dizier, B.; Bacha, N.; Lokajczyk, A.; Guerin, C.L.; Gendron, N.; Planquette, B.; et al. Co-injection of mesenchymal stem cells with endothelial progenitor cells accelerates muscle recovery in hind limb ischemia through an endoglin-dependent mechanism. Thromb. Haemost. 2017, 117, 1908–1918.Le Ricousse-Roussanne, S.; Barateau, V.; Contreres, J.O.; Boval, B.; Kraus-Berthier, L.; Tobelem, G. Ex vivo differentiated endothelial and smooth muscle cells from human cord blood progenitors home to the angiogenic tumor vasculature. Cardiovasc. Res. 2004, 62, 176–184.
- Foubert, P.; Matrone, G.; Souttou, B.; Lere-Dean, C.; Barateau, V.; Plouet, J.; Le Ricousse-Roussanne, S.; Levy, B.I.; Silvestre, J.S.; Tobelem, G. Coadministration of endothelial and smooth muscle progenitor cells enhances the efficiency of proangiogenic cell-based therapy. Circ. Res. 2008, 103, 751–760.Fowkes, F.G.; Aboyans, V.; Fowkes, F.J.; McDermott, M.M.; Sampson, U.K.; Criqui, M.H. Peripheral artery disease: Epidemiology and global perspectives. Nat. Rev. Cardiol. 2017, 14, 156–170.
- Le Ricousse-Roussanne, S.; Barateau, V.; Contreres, J.O.; Boval, B.; Kraus-Berthier, L.; Tobelem, G. Ex vivo differentiated endothelial and smooth muscle cells from human cord blood progenitors home to the angiogenic tumor vasculature. Cardiovasc. Res. 2004, 62, 176–184.Rojas-Torres, M.; Jiménez-Palomares, M.; Martín-Ramírez, J.; Beltrán-Camacho, L.; Sánchez-Gomar, I.; Eslava-Alcon, S.; Rosal-Vela, A.; Gavaldá, S.; Durán-Ruiz, M.C. REX-001, a BM-MNC Enriched Solution, Induces Revascularization of Ischemic Tissues in a Murine Model of Chronic Limb-Threatening Ischemia. Front. Cell Dev. Biol. 2020, 8, 1546.
- Fowkes, F.G.; Aboyans, V.; Fowkes, F.J.; McDermott, M.M.; Sampson, U.K.; Criqui, M.H. Peripheral artery disease: Epidemiology and global perspectives. Nat. Rev. Cardiol. 2017, 14, 156–170.Ratajczak, M.Z.; Zuba-Surma, E.K.; Machalinski, B.; Ratajczak, J.; Kucia, M. Very small embryonic-like (VSEL) stem cells: Purification from adult organs, characterization, and biological significance. Stem. Cell Rev. 2008, 4, 89–99.
- Rojas-Torres, M.; Jiménez-Palomares, M.; Martín-Ramírez, J.; Beltrán-Camacho, L.; Sánchez-Gomar, I.; Eslava-Alcon, S.; Rosal-Vela, A.; Gavaldá, S.; Durán-Ruiz, M.C. REX-001, a BM-MNC Enriched Solution, Induces Revascularization of Ischemic Tissues in a Murine Model of Chronic Limb-Threatening Ischemia. Front. Cell Dev. Biol. 2020, 8, 1546.Amann, B.; Luedemann, C.; Ratei, R.; Schmidt-Lucke, J.A. Autologous bone marrow cell transplantation increases leg perfusion and reduces amputations in patients with advanced critical limb ischemia due to peripheral artery disease. Cell Transplant. 2009, 18, 371–380.
- Ratajczak, M.Z.; Zuba-Surma, E.K.; Machalinski, B.; Ratajczak, J.; Kucia, M. Very small embryonic-like (VSEL) stem cells: Purification from adult organs, characterization, and biological significance. Stem. Cell Rev. 2008, 4, 89–99.Fadini, G.P.; Agostini, C.; Avogaro, A. Autologous stem cell therapy for peripheral arterial disease meta-analysis and systematic review of the literature. Atherosclerosis 2010, 209, 10–17.
- Amann, B.; Luedemann, C.; Ratei, R.; Schmidt-Lucke, J.A. Autologous bone marrow cell transplantation increases leg perfusion and reduces amputations in patients with advanced critical limb ischemia due to peripheral artery disease. Cell Transplant. 2009, 18, 371–380.Guo, J.; Guo, L.; Cui, S.; Tong, Z.; Dardik, A.; Gu, Y. Autologous bone marrow-derived mononuclear cell therapy in Chinese patients with critical limb ischemia due to thromboangiitis obliterans: 10-year results. Stem. Cell Res. Ther. 2018, 9, 43.
- Fadini, G.P.; Agostini, C.; Avogaro, A. Autologous stem cell therapy for peripheral arterial disease meta-analysis and systematic review of the literature. Atherosclerosis 2010, 209, 10–17.Idei, N.; Soga, J.; Hata, T.; Fujii, Y.; Fujimura, N.; Mikami, S.; Maruhashi, T.; Nishioka, K.; Hidaka, T.; Kihara, Y.; et al. Limb ischemia: A comparison of atherosclerotic peripheral arterial disease and Buerger disease. Circ. Cardiovasc. Interv. 2011, 4, 15–25.
- Guo, J.; Guo, L.; Cui, S.; Tong, Z.; Dardik, A.; Gu, Y. Autologous bone marrow-derived mononuclear cell therapy in Chinese patients with critical limb ischemia due to thromboangiitis obliterans: 10-year results. Stem. Cell Res. Ther. 2018, 9, 43.Liang, T.W.; Jester, A.; Motaganahalli, R.L.; Wilson, M.G.; G’Sell, P.; Akingba, G.A.; Fajardo, A.; Murphy, M.P. Autologous bone marrow mononuclear cell therapy for critical limb ischemia is effective and durable. J. Vasc. Surg. 2016, 63, 1541–1545.
- Idei, N.; Soga, J.; Hata, T.; Fujii, Y.; Fujimura, N.; Mikami, S.; Maruhashi, T.; Nishioka, K.; Hidaka, T.; Kihara, Y.; et al. Limb ischemia: A comparison of atherosclerotic peripheral arterial disease and Buerger disease. Circ. Cardiovasc. Interv. 2011, 4, 15–25.Murphy, M.P.; Lawson, J.H.; Rapp, B.M.; Dalsing, M.C.; Klein, J.; Wilson, M.G.; Hutchins, G.D.; March, K.L. Autologous bone marrow mononuclear cell therapy is safe and promotes amputation-free survival in patients with critical limb ischemia. J. Vasc. Surg. 2011, 53, 1565–1574 e1.
- Liang, T.W.; Jester, A.; Motaganahalli, R.L.; Wilson, M.G.; G’Sell, P.; Akingba, G.A.; Fajardo, A.; Murphy, M.P. Autologous bone marrow mononuclear cell therapy for critical limb ischemia is effective and durable. J. Vasc. Surg. 2016, 63, 1541–1545.Ruiz-Salmeron, R.; de la Cuesta-Diaz, A.; Constantino-Bermejo, M.; Perez-Camacho, I.; Marcos-Sanchez, F.; Hmadcha, A.; Soria, B. Angiographic demonstration of neoangiogenesis after intra-arterial infusion of autologous bone marrow mononuclear cells in diabetic patients with critical limb ischemia. Cell Transplant. 2011, 20, 1629–1639.
- Murphy, M.P.; Lawson, J.H.; Rapp, B.M.; Dalsing, M.C.; Klein, J.; Wilson, M.G.; Hutchins, G.D.; March, K.L. Autologous bone marrow mononuclear cell therapy is safe and promotes amputation-free survival in patients with critical limb ischemia. J. Vasc. Surg. 2011, 53, 1565–1574 e1.Wahid, F.S.A.; Ismail, N.A.; Wan Jamaludin, W.F.; Muhamad, N.A.; Mohamad Idris, M.A.; Lai, N.M. Efficacy and Safety of Autologous Cell-based Therapy in Patients with No-option Critical Limb Ischaemia: A Meta-Analysis. Curr. Stem. Cell Res. Ther. 2018, 13, 265–283.
- Ruiz-Salmeron, R.; de la Cuesta-Diaz, A.; Constantino-Bermejo, M.; Perez-Camacho, I.; Marcos-Sanchez, F.; Hmadcha, A.; Soria, B. Angiographic demonstration of neoangiogenesis after intra-arterial infusion of autologous bone marrow mononuclear cells in diabetic patients with critical limb ischemia. Cell Transplant. 2011, 20, 1629–1639.Yusoff, F.M.; Kajikawa, M.; Matsui, S.; Hashimoto, H.; Kishimoto, S.; Maruhashi, T.; Chowdhury, M.; Noma, K.; Nakashima, A.; Kihara, Y.; et al. Review of the Long-term Effects of Autologous Bone-Marrow Mononuclear Cell Implantation on Clinical Outcomes in Patients with Critical Limb Ischemia. Sci. Rep. 2019, 9, 7711.
- Wahid, F.S.A.; Ismail, N.A.; Wan Jamaludin, W.F.; Muhamad, N.A.; Mohamad Idris, M.A.; Lai, N.M. Efficacy and Safety of Autologous Cell-based Therapy in Patients with No-option Critical Limb Ischaemia: A Meta-Analysis. Curr. Stem. Cell Res. Ther. 2018, 13, 265–283.Kumar, A.; Prasad, M.; Jali, V.P.; Pandit, A.K.; Misra, S.; Kumar, P.; Chakravarty, K.; Kathuria, P.; Gulati, A. Bone marrow mononuclear cell therapy in ischaemic stroke: A systematic review. Acta. Neurol. Scand. 2017, 135, 496–506.
- Yusoff, F.M.; Kajikawa, M.; Matsui, S.; Hashimoto, H.; Kishimoto, S.; Maruhashi, T.; Chowdhury, M.; Noma, K.; Nakashima, A.; Kihara, Y.; et al. Review of the Long-term Effects of Autologous Bone-Marrow Mononuclear Cell Implantation on Clinical Outcomes in Patients with Critical Limb Ischemia. Sci. Rep. 2019, 9, 7711.Shintani, S.; Murohara, T.; Ikeda, H.; Ueno, T.; Sasaki, K.; Duan, J.; Imaizumi, T. Augmentation of postnatal neovascularization with autologous bone marrow transplantation. Circulation 2001, 103, 897–903.
- Kumar, A.; Prasad, M.; Jali, V.P.; Pandit, A.K.; Misra, S.; Kumar, P.; Chakravarty, K.; Kathuria, P.; Gulati, A. Bone marrow mononuclear cell therapy in ischaemic stroke: A systematic review. Acta. Neurol. Scand. 2017, 135, 496–506.Kikuchi-Taura, A.; Okinaka, Y.; Takeuchi, Y.; Ogawa, Y.; Maeda, M.; Kataoka, Y.; Yasui, T.; Kimura, T.; Gul, S.; Claussen, C.; et al. Bone Marrow Mononuclear Cells Activate Angiogenesis via Gap Junction-Mediated Cell-Cell Interaction. Stroke 2020, 51, 1279–1289.
- Shintani, S.; Murohara, T.; Ikeda, H.; Ueno, T.; Sasaki, K.; Duan, J.; Imaizumi, T. Augmentation of postnatal neovascularization with autologous bone marrow transplantation. Circulation 2001, 103, 897–903.Huang, P.; Li, S.; Han, M.; Xiao, Z.; Yang, R.; Han, Z.C. Autologous transplantation of granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cells improves critical limb ischemia in diabetes. Diabetes. Care 2005, 28, 2155–2160.
- Kikuchi-Taura, A.; Okinaka, Y.; Takeuchi, Y.; Ogawa, Y.; Maeda, M.; Kataoka, Y.; Yasui, T.; Kimura, T.; Gul, S.; Claussen, C.; et al. Bone Marrow Mononuclear Cells Activate Angiogenesis via Gap Junction-Mediated Cell-Cell Interaction. Stroke 2020, 51, 1279–1289.Kawamura, A.; Horie, T.; Tsuda, I.; Abe, Y.; Yamada, M.; Egawa, H.; Iida, J.; Sakata, H.; Onodera, K.; Tamaki, T.; et al. Clinical study of therapeutic angiogenesis by autologous peripheral blood stem cell (PBSC) transplantation in 92 patients with critically ischemic limbs. J. Artif. Organs. 2006, 9, 226–233.
- Huang, P.; Li, S.; Han, M.; Xiao, Z.; Yang, R.; Han, Z.C. Autologous transplantation of granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cells improves critical limb ischemia in diabetes. Diabetes. Care 2005, 28, 2155–2160.Li, S.; Zhou, B.; Han, Z.C. Therapeutic neovascularization by transplantation of mobilized peripheral blood mononuclear cells for limb ischemia. A comparison between CD34+ and CD34- mononuclear cells. Thromb. Haemost. 2006, 95, 301–311.
- Kawamura, A.; Horie, T.; Tsuda, I.; Abe, Y.; Yamada, M.; Egawa, H.; Iida, J.; Sakata, H.; Onodera, K.; Tamaki, T.; et al. Clinical study of therapeutic angiogenesis by autologous peripheral blood stem cell (PBSC) transplantation in 92 patients with critically ischemic limbs. J. Artif. Organs. 2006, 9, 226–233.Padilla, L.; Arguero-Sanchez, R.; Rodriguez-Trejo, J.M.; Carranza-Castro, P.H.; Suarez-Cuenca, J.A.; Polaco-Castillo, J.; DiSilvio-Lopez, M.; Lopez-Gutierrez, J.; Olguin-Juarez, H.; Hernandez-Patricio, A.; et al. Effect of autologous transplant of peripheral blood mononuclear cells in combination with proangiogenic factors during experimental revascularization of lower limb ischemia. J. Tissue. Eng. Regen. Med. 2020, 14, 600–608.
- Li, S.; Zhou, B.; Han, Z.C. Therapeutic neovascularization by transplantation of mobilized peripheral blood mononuclear cells for limb ischemia. A comparison between CD34+ and CD34- mononuclear cells. Thromb. Haemost. 2006, 95, 301–311.Mohammadzadeh, L.; Samedanifard, S.H.; Keshavarzi, A.; Alimoghaddam, K.; Larijani, B.; Ghavamzadeh, A.; Ahmadi, A.S.; Shojaeifard, A.; Ostadali, M.R.; Sharifi, A.M.; et al. Therapeutic outcomes of transplanting autologous granulocyte colony-stimulating factor-mobilised peripheral mononuclear cells in diabetic patients with critical limb ischaemia. Exp. Clin. Endocrinol. Diabetes 2013, 121, 48–53.
- Padilla, L.; Arguero-Sanchez, R.; Rodriguez-Trejo, J.M.; Carranza-Castro, P.H.; Suarez-Cuenca, J.A.; Polaco-Castillo, J.; DiSilvio-Lopez, M.; Lopez-Gutierrez, J.; Olguin-Juarez, H.; Hernandez-Patricio, A.; et al. Effect of autologous transplant of peripheral blood mononuclear cells in combination with proangiogenic factors during experimental revascularization of lower limb ischemia. J. Tissue. Eng. Regen. Med. 2020, 14, 600–608.Ozturk, A.; Kucukardali, Y.; Tangi, F.; Erikci, A.; Uzun, G.; Bashekim, C.; Sen, H.; Terekeci, H.; Narin, Y.; Ozyurt, M.; et al. Therapeutical potential of autologous peripheral blood mononuclear cell transplantation in patients with type 2 diabetic critical limb ischemia. J. Diabetes Complicat. 2012, 26, 29–33.
- Mohammadzadeh, L.; Samedanifard, S.H.; Keshavarzi, A.; Alimoghaddam, K.; Larijani, B.; Ghavamzadeh, A.; Ahmadi, A.S.; Shojaeifard, A.; Ostadali, M.R.; Sharifi, A.M.; et al. Therapeutic outcomes of transplanting autologous granulocyte colony-stimulating factor-mobilised peripheral mononuclear cells in diabetic patients with critical limb ischaemia. Exp. Clin. Endocrinol. Diabetes 2013, 121, 48–53.Dubsky, M.; Jirkovska, A.; Bem, R.; Fejfarova, V.; Pagacova, L.; Sixta, B.; Varga, M.; Langkramer, S.; Sykova, E.; Jude, E.B. Both autologous bone marrow mononuclear cell and peripheral blood progenitor cell therapies similarly improve ischaemia in patients with diabetic foot in comparison with control treatment. Diabetes Metab. Res. Rev. 2013, 29, 369–376.
- Ozturk, A.; Kucukardali, Y.; Tangi, F.; Erikci, A.; Uzun, G.; Bashekim, C.; Sen, H.; Terekeci, H.; Narin, Y.; Ozyurt, M.; et al. Therapeutical potential of autologous peripheral blood mononuclear cell transplantation in patients with type 2 diabetic critical limb ischemia. J. Diabetes Complicat. 2012, 26, 29–33.Minamino, T.; Toko, H.; Tateno, K.; Nagai, T.; Komuro, I. Peripheral-blood or bone-marrow mononuclear cells for therapeutic angiogenesis? Lancet 2002, 360, 2083–2084; author reply 2084.
- Dubsky, M.; Jirkovska, A.; Bem, R.; Fejfarova, V.; Pagacova, L.; Sixta, B.; Varga, M.; Langkramer, S.; Sykova, E.; Jude, E.B. Both autologous bone marrow mononuclear cell and peripheral blood progenitor cell therapies similarly improve ischaemia in patients with diabetic foot in comparison with control treatment. Diabetes Metab. Res. Rev. 2013, 29, 369–376.Capiod, J.C.; Tournois, C.; Vitry, F.; Sevestre, M.A.; Daliphard, S.; Reix, T.; Nguyen, P.; Lefrere, J.J.; Pignon, B. Characterization and comparison of bone marrow and peripheral blood mononuclear cells used for cellular therapy in critical leg ischaemia: Towards a new cellular product. Vox. Sang. 2009, 96, 256–265.
- Minamino, T.; Toko, H.; Tateno, K.; Nagai, T.; Komuro, I. Peripheral-blood or bone-marrow mononuclear cells for therapeutic angiogenesis? Lancet 2002, 360, 2083–2084; author reply 2084.Mazini, L.; Rochette, L.; Amine, M.; Malka, G. Regenerative Capacity of Adipose Derived Stem Cells (ADSCs), Comparison with Mesenchymal Stem Cells (MSCs). Int. J. Mol. Sci. 2019, 20, 2523.
- Capiod, J.C.; Tournois, C.; Vitry, F.; Sevestre, M.A.; Daliphard, S.; Reix, T.; Nguyen, P.; Lefrere, J.J.; Pignon, B. Characterization and comparison of bone marrow and peripheral blood mononuclear cells used for cellular therapy in critical leg ischaemia: Towards a new cellular product. Vox. Sang. 2009, 96, 256–265.Rehman, J.; Traktuev, D.; Li, J.; Merfeld-Clauss, S.; Temm-Grove, C.J.; Bovenkerk, J.E.; Pell, C.L.; Johnstone, B.H.; Considine, R.V.; March, K.L. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 2004, 109, 1292–1298.
- Mazini, L.; Rochette, L.; Amine, M.; Malka, G. Regenerative Capacity of Adipose Derived Stem Cells (ADSCs), Comparison with Mesenchymal Stem Cells (MSCs). Int. J. Mol. Sci. 2019, 20, 2523.Safford, K.M.; Hicok, K.C.; Safford, S.D.; Halvorsen, Y.D.; Wilkison, W.O.; Gimble, J.M.; Rice, H.E. Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem. Biophys. Res. Commun. 2002, 294, 371–379.
- Rehman, J.; Traktuev, D.; Li, J.; Merfeld-Clauss, S.; Temm-Grove, C.J.; Bovenkerk, J.E.; Pell, C.L.; Johnstone, B.H.; Considine, R.V.; March, K.L. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 2004, 109, 1292–1298.Dragoo, J.L.; Choi, J.Y.; Lieberman, J.R.; Huang, J.; Zuk, P.A.; Zhang, J.; Hedrick, M.H.; Benhaim, P. Bone induction by BMP-2 transduced stem cells derived from human fat. J. Orthop. Res. 2003, 21, 622–629.
- Safford, K.M.; Hicok, K.C.; Safford, S.D.; Halvorsen, Y.D.; Wilkison, W.O.; Gimble, J.M.; Rice, H.E. Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem. Biophys. Res. Commun. 2002, 294, 371–379.Zhi, K.; Gao, Z.; Bai, J.; Wu, Y.; Zhou, S.; Li, M.; Qu, L. Application of adipose-derived stem cells in critical limb ischemia. Front Biosci. 2014, 19, 768–776.
- Dragoo, J.L.; Choi, J.Y.; Lieberman, J.R.; Huang, J.; Zuk, P.A.; Zhang, J.; Hedrick, M.H.; Benhaim, P. Bone induction by BMP-2 transduced stem cells derived from human fat. J. Orthop. Res. 2003, 21, 622–629.Liu, J.; Qiu, P.; Qin, J.; Wu, X.; Wang, X.; Yang, X.; Li, B.; Zhang, W.; Ye, K.; Peng, Z.; et al. Allogeneic adipose-derived stem cells promote ischemic muscle repair by inducing M2 macrophage polarization via the HIF-1alpha/IL-10 pathway. Stem. Cells 2020, 38, 1307–1320.
- Zhi, K.; Gao, Z.; Bai, J.; Wu, Y.; Zhou, S.; Li, M.; Qu, L. Application of adipose-derived stem cells in critical limb ischemia. Front Biosci. 2014, 19, 768–776.Zahavi-Goldstein, E.; Blumenfeld, M.; Fuchs-Telem, D.; Pinzur, L.; Rubin, S.; Aberman, Z.; Sher, N.; Ofir, R. Placenta-derived PLX-PAD mesenchymal-like stromal cells are efficacious in rescuing blood flow in hind limb ischemia mouse model by a dose- and site-dependent mechanism of action. Cytotherapy 2017, 19, 1438–1446.
- Han, S.; Sun, H.M.; Hwang, K.C.; Kim, S.W. Adipose-Derived Stromal Vascular Fraction Cells: Update on Clinical Utility and Efficacy. Crit. Rev. Eukaryot. Gene. Expr. 2015, 25, 145–152.Hong, S.J.; Traktuev, D.O.; March, K.L. Therapeutic potential of adipose-derived stem cells in vascular growth and tissue repair. Curr. Opin. Organ. Transplant. 2010, 15, 86–91.
- Liu, J.; Qiu, P.; Qin, J.; Wu, X.; Wang, X.; Yang, X.; Li, B.; Zhang, W.; Ye, K.; Peng, Z.; et al. Allogeneic adipose-derived stem cells promote ischemic muscle repair by inducing M2 macrophage polarization via the HIF-1alpha/IL-10 pathway. Stem. Cells 2020, 38, 1307–1320.Katagiri, T.; Kondo, K.; Shibata, R.; Hayashida, R.; Shintani, S.; Yamaguchi, S.; Shimizu, Y.; Unno, K.; Kikuchi, R.; Kodama, A.; et al. Therapeutic angiogenesis using autologous adipose-derived regenerative cells in patients with critical limb ischaemia in Japan: A clinical pilot study. Sci. Rep. 2020, 10, 16045.
- Hong, S.J.; Traktuev, D.O.; March, K.L. Therapeutic potential of adipose-derived stem cells in vascular growth and tissue repair. Curr. Opin. Organ. Transplant. 2010, 15, 86–91.
- Katagiri, T.; Kondo, K.; Shibata, R.; Hayashida, R.; Shintani, S.; Yamaguchi, S.; Shimizu, Y.; Unno, K.; Kikuchi, R.; Kodama, A.; et al. Therapeutic angiogenesis using autologous adipose-derived regenerative cells in patients with critical limb ischaemia in Japan: A clinical pilot study. Sci. Rep. 2020, 10, 16045.
- Zahavi-Goldstein, E.; Blumenfeld, M.; Fuchs-Telem, D.; Pinzur, L.; Rubin, S.; Aberman, Z.; Sher, N.; Ofir, R. Placenta-derived PLX-PAD mesenchymal-like stromal cells are efficacious in rescuing blood flow in hind limb ischemia mouse model by a dose- and site-dependent mechanism of action. Cytotherapy 2017, 19, 1438–1446.
