ω3- fatty acids (ω3-FAs) such as docosahexaenoic acid (DHA, 22:6ω-3) and eicosapentaenoic acid (EPA, 20:5ω-3) have drawn attention over the last decades because their consumption is related to several beneficial effects on human health. Seafood is a traditional source of ω-3 FAs but the recovery of these bioactive substances from fishery waste represents an interesting alternative, allowing to benefit both the environment and the global economy through the valorization of rest raw materials.
- omega-3
- fish waste
- supplements
- fish oil extraction
1. Introduction
The exploitation of food of both plant and animal origin for the recovery of added-value products represents an attractive opportunity to implement sustainable practices in waste management [1]. Fish leftovers and processing byproducts (e.g., non-edible parts and damaged whole fish) contribute to global waste, representing from 20 to 80% of the original fish [2][3]. Its heterogeneity allows the retrieval of a wide range of compounds including lipids. Fish lipids are currently being extracted from several species, such as cod, herring, mackerel, tuna, salmon, anchovy, menhaden, and sardine [4].
Fish lipid composition depends on a multitude of factors, with food availability being a major determinant. Other differences may arise from geographic location, type of water (marine or freshwater), season, and maturity of the fish. Lipids are mainly present in fish oil as triacylglycerols (TAGs), fatty acid esters of glycerol (Figure 1) [4].
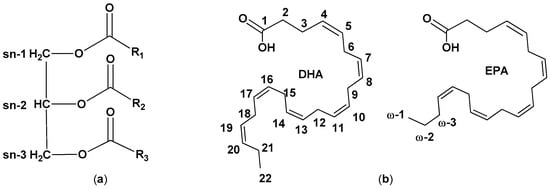
Figure 1.
(
a
) Simplified structure of triacylglycerol and (
b
) chemical structure of and docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA).
A minor number of FAs occur as phospholipids, amphipathic molecules containing polar head groups linked to the glycerol moiety via phosphate linkage. Different classes of FAs are present in lipids: saturated FAs (SFAs), monounsaturated FAs (MFAs, containing one C-C double bond), and polyunsaturated FAs (PUFAs, containing more than one C-C double bonds). FAs are also classed according to the position of the first double bond counting from the terminal omega (ω) carbon: ω-3 FAs have the first double bond on carbon 3. Among PUFAs, ω-3 FAs such as docosahexaenoic acid (DHA, 22:6ω-3) and eicosapentaenoic acid (EPA, 20:5ω-3) have received attention because they are claimed to have a preventive role in the development and progression of a broad range of conditions, such as cardiovascular diseases (CVDs) [5][6][7]. Despite their role is stile debated, their benefits are reported also based on some randomized clinical trials, so ω-3 FAs supplements are sometimes recommended when a sufficient intake through fish consumption is impossible. The increased demand for ω3-FAs urged the search for alternative and sustainable sources, thus reducing the pressure on the marine ecosystem. Fish oil can contain up to ca. 50% of omega-3 PUFAs, with EPA and DHA accounting from 5 to 26 w/w% of total FAs The oil distribution in the tissues is usually heterogeneous and different byproducts contain different amounts of FAs [8][9].
2. Critical issues in omega-3 fatty acids recovery from fish waste
Degradation phenomena and the possible presence of contaminants pose challenges to the recovery of so ω-3 FAs from fish waste. The elevated content in water and nutrients make fish leftovers a good growth medium for bacteria and fungi that can be toxic and/or impair their nutritional value, while the high content in PUFAs makes this type of waste highly susceptible to lipid peroxidation, a free-radical mediated oxidative process typically triggered by heat, light, metal, peroxide, or hydroperoxide. A complex mixture of volatile and non-volatile compounds with different molecular weights, polarity, and functional groups is formed, including aldehydes and ketones, responsible for the bad smell and off-flavors of rancid oil.
Despite some oxidation products may be beneficial to health, others are related to aging, mutagenesis, and carcinogenesis [10][11]. Broadly speaking, lipid peroxidation impairs the quality of the oil and its nutritional value, causes darkening, off-flavors, and pronounced unpleasant odors. Fish oil proneness to oxidative processes imposes special precautions covering both production and storage and affects shelf life, nutritional value, safety, and consumers’ acceptability [10].
Light and oxygen exposure, prolonged heating, and pro-oxidants, such as transition metals should be avoided because they accelerate lipid oxidation. Waste and oils should be immediately stored at −20 °C in amber glass or Teflon sealed containers, trying to minimize air contact, possibly, under a nitrogen atmosphere. The addition of natural or synthetic antioxidant compounds that prevent or retard oxidative deteriorative processes may be necessary.
Marine pollution results in the presence of potentially toxic substances such as heavy metals (and metalloids), pesticides, polychlorinated biphenyls (PCBs), and dioxins in fish oils [12]. These fat-soluble contaminants accumulate and have a huge impact, especially when considering oil from predatory fish, on the top position of the food chain.
Heavy metals such as mercury, cadmium, lead, and arsenic pose a threat to human health causing several adverse effects on a number of organs, while dioxins and PCBs are known to be carcinogenic to humans and have been related to reproductive disorders, neurological problems, and other diseases [13].
To maximize health benefits and minimize potential detrimental effects, extraction, and purification processes should reduce contaminants in the final product without impacting the quality of the oil [14]. Decontamination efficiency is variable and monitoring these contaminants in fish oil supplements is recommended to evaluate their possible contribution to total human exposure.
3. Fish oil extraction and omega-3 concentration
Traditional lipid extraction techniques involve the use of organic solvents but due to the restriction in using organic solvents in the food industry and to the research of environmentally friendly and sustainable processes, alternative strategies have been proposed.
On an industrial scale, fish oil extraction is commonly carried out by a process known as “wet reduction” (or “wet rendering”) (
Figure 2
).
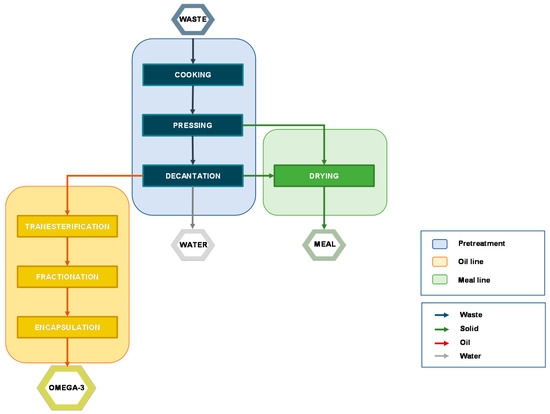
Figure 2.
Block flow diagram (BFD) of a typical process from fish waste to final products.
This process separates the three main fraction the fish waste is made of (solid, water, and oil) through different operations, including:
-
Cooking: this step coagulates protein and induces the rupture of the cell walls, favoring the release of oil and water. The temperature should not exceed 90-100 °C (for 15-20 mins) because overcooking may promote lipid peroxidation.
Pressing/Centrifugation: the liquid fraction is separated from the solid one that is typically used to produce fishmeal.
Decantation:
this step allows the separation of oil and water fraction. Being a slow operation, it has been often overtaken by centrifugation.
Drying:
the solid fraction is disintegrated, mixed, and heated at high temperature (ca. 100 °C) to ease the removal of water and prevent microbial activity. The resulting proteinaceous flour-type material is used as animal feed.
Wet rendering is an energy-intensive process and may prompt the degradation of heat-sensitive PUFAs. Consequently, alternative methods are sometimes considered to extract fish oil from waste. These include microwave-assisted extraction (MAE), ultrasound-assisted extraction (UAE), enzymatic methods, and the use of supercritical fluids (SFs) [15].
MAE uses microwaves to warm solvents in contact with the biomass reducing its moisture and resulting in increased pressure that disrupts the cell membranes of the fish, speeding up the process. UAE exploits acoustic cavitation to break the cell walls, improving the penetration of the solvent and easing the release of the oil. Enzymatic methods require neither organic solvents nor high temperature and involve the use of exogenous proteolytic enzymes (e.g., protease, exopeptidase, or endopeptidase) to remove lipids from fish waste. These methods are in line with green chemistry practices: enzymes are reusable, there is no need for toxic solvents or extreme temperature conditions, and byproducts production is limited. However, the oil usually needs further treatments to remove some undesired components and the scale-up may be difficult[16].
A supercritical solvent is a fluid that under specific conditions of temperature and pressure (in the region above its critical point) shows unique properties: density-wise it is similar to a liquid while its viscosity, diffusivity, and compressibility are similar to the gas ones. Since the polarity of SFs increases with density, and because of the variation of density with pressure and temperature, tuning these two parameters modulates SCF solvent power allowing the extraction and/or concentration of a wide range of substances [17]. The most used SCF is scCO2 because its critical conditions are relatively easy to access (Tc = 304.15 K and Pc = 7.38 MPa). It is considered safe because non-toxic, non-corrosive, and non-flammable. Moreover, it can be easily removed after the extraction because it is gaseous and leaves no traces in the product. Operating at low temperatures, this technique is suitable for thermolabile compounds, while the displacement of the oxygen by the scCO2 contributes to preserving ω-3 FAs from oxidation [18]. Despite being a promising method, it has not replaced the wet rendering method on an industrial scale, partly due to the high initial investment.
The production of oil suitable for food consumption requires the removal of undesired compounds that may affect consumer acceptability (e.g., smelly or colored substances) and sometimes be toxic (e.g., heavy metals, dioxins, or polychlorinated biphenyls) [13][17]. Steps that are usually included are the following: degumming (removal of phospholipids), neutralization (clearing of free FAs), bleaching (absorption of pigments, trace metals, oxidation products, and other contaminants), and deodorization (removal of smelly compounds).
All these traditional refining processes involve the use of chemicals that affects their environmental impact or require high temperatures that are not suitable for thermolabile compounds. Environmental-friendly alternative options have been proposed. Supercritical fluid technology has been used to remove some undesired compounds like free FAs or toxic compounds (e.g., dioxins), and to degum or bleach the oil as well [17].
Different purification techniques may require to be preceded by different processing procedures, but a typical way to improve purification yield is oil transesterification [19]. Since FAs are preferentially bound at the central position of TAGs, to achieve a high grade of purity is necessary to free FAs from the glycerol backbone. On a large scale, transesterification is usually carried out using strong acid or alkaline catalysts, but the need for more environmental-friendly methods has prompted the introduction and the development of alternative technologies, such as enzymatic methods.
Once the oil has been extracted, ω-3 FAs must be concentrated to match the commercial demand for concentrated fish oil (from 60 to 90% by weight). Fractionation can be performed using different technologies depending on material composition, target purity, selectivity, stability, and environmental consideration. Some commonly used methodologies are molecular distillation (MD), urea precipitation, enzymatic enrichment, and supercritical fluid techniques (or a combination of two or more of them). Even if MD is the most utilized method to concentrate ω-3 FAs at an industrial scale, supercritical techniques could replace it because of their higher selectivity and lower operating temperatures, which minimizes the risk for oxidation.
4. Omega-3 formulations
Omega-3 FAs can be present in several forms in supplements, including natural TAGs, ethyl esters, free fatty acids, phospholipids, and re-esterified TAG enriched in ω-3 FAs. The conversion of ω-3 ethyl esters back into triglycerides form raises from the observation that ω-3 FAs absorption seems to be faster and higher when TAGs are consumed [18][20]. To improve ω-3 FAs absorption and bioavailability and preserve them from oxidation, proper formulation and delivery system are required. The selective delivery and the controlled release of an active compound not only does allow the release of the ω-3 FAs directly in the intestine but also protect lipids from oxidation, extending shelf-life and masking taste and smell [21].
Encapsulation of ω-3 FAs is currently achieved by different techniques. Among classical methods, spray-drying and coacervation are widely used also on a large scale. The selection of the more appropriate method depends on the interaction of the solvent with the compound of interest, the desired morphology, the particle size distribution, the coating material, and the solvents used (if any) [22][23][24]. Other parameters to be evaluated are the encapsulation efficiency and the residual solvent concentration.
Traditional micronization and encapsulation techniques do not usually allow a high level of control of particle size distribution. Moreover, they often use toxic and polluting solvents and need high temperature, unsuitable for thermolabile compounds. Other techniques have been proposed to overcome these problems, such as the freeze-drying of emulsions, the encapsulation of omega-3 FAs in the lipid vesicular system on the micro- and nano-scale, or the use of supercritical fluids. Four main SFC micronization variants do exist: supercritical solvent precipitation, supercritical antisolvent precipitation, supercritical fluid as a solute, methods based on drying technologies [25][22].
5. Conclusions
Fish processing and farming can generate considerable amounts of leftovers and byproducts that negatively affect the environment. Some of this waste can be efficiently converted into marketable high-value products, representing a more interesting option than their non-utilization or disposal towards open waters or landfills. Fish oil-derived dietary supplements are widely marketed across the world and this urges the development of responsible strategies to utilize resources that are renewable but not infinite. The efficient minimization and exploitation of fish waste and its transformation into high-value products is an attractive solution both from an environmental and economic point of view.
Despite undeniable signs of progress, great room for improvement exists, making essential the promotion of sustainable practices sustained by technological innovation and government policies. Further research is necessary to obtain highly pure, stable, safe, and bioavailable ω-3 concentrates, while clear communication of improvements in sustainability along with the safety of products obtained from waste is essential to consumers’ acceptability. Waste should not be considered less valuable than the fish itself but a precious and profitable resource capable of bringing health, social, economic, and environmental benefits.
References
- Antonietta Baiano; Recovery of Biomolecules from Food Wastes — A Review. Molecules 2014, 19, 14821-14842, 10.3390/molecules190914821.
- Ishita Ahuja; Egidijus Dauksas; Jannicke F. Remme; Roger Richardsen; Anne-Kristin Løes; Fish and fish waste-based fertilizers in organic farming – With status in Norway: A review. Waste Management 2020, 115, 95-112, 10.1016/j.wasman.2020.07.025.
- Lukas Kratky; Petr Zamazal; Economic feasibility and sensitivity analysis of fish waste processing biorefinery. Journal of Cleaner Production 2020, 243, 118677, 10.1016/j.jclepro.2019.118677.
- Huijun Zhang; Hui Zhao; Youwei Zhang; Yingbin Shen; Hang Su; Jun Jin; Qingzhe Jin; Xingguo Wang; Characterization of Positional Distribution of Fatty Acids and Triacylglycerol Molecular Compositions of Marine Fish Oils Rich in Omega-3 Polyunsaturated Fatty Acids. BioMed Research International 2018, 2018, 1-10, 10.1155/2018/3529682.
- Asmaa S Abdelhamid; Tracey J Brown; Julii S Brainard; Priti Biswas; Gabrielle C Thorpe; Helen J Moore; Katherine Ho Deane; Carolyn D Summerbell; Helen V Worthington; Fujian Song; et al.Lee Hooper Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews 2020, 3, CD003177, 10.1002/14651858.cd003177.pub5.
- JoAnn E. Manson; Shari S. Bassuk; Nancy R. Cook; I-Min Lee; Samia Mora; Christine M. Albert; Julie E. Buring; for the VITAL Research Group; Vitamin D, Marine n-3 Fatty Acids, and Primary Prevention of Cardiovascular Disease Current Evidence. Circulation Research 2020, 126, 112-128, 10.1161/circresaha.119.314541.
- Jacqueline F Gould; Lisa G Smithers; Maria Makrides; The effect of maternal omega-3 (n-3) LCPUFA supplementation during pregnancy on early childhood cognitive and visual development: a systematic review and meta-analysis of randomized controlled trials. The American Journal of Clinical Nutrition 2013, 97, 531-544, 10.3945/ajcn.112.045781.
- F. Sahena; I. S. M. Zaidul; S. Jinap; N. Saari; H. A. Jahurul; K. A. Abbas; N. A. Norulaini; PUFAs in Fish: Extraction, Fractionation, Importance in Health. Comprehensive Reviews in Food Science and Food Safety 2009, 8, 59-74, 10.1111/j.1541-4337.2009.00069.x.
- Mustafa Durmuş; Fish oil for human health: omega-3 fatty acid profiles of marine seafood species. Food Science and Technology 2019, 39, 454-461, 10.1590/fst.21318.
- Elmira Arab-Tehrany; Muriel Jacquot; Claire Gaiani; Muhammad Imran; Stephane Desobry; Michel Linder; Beneficial effects and oxidative stability of omega-3 long-chain polyunsaturated fatty acids. Trends in Food Science & Technology 2012, 25, 24-33, 10.1016/j.tifs.2011.12.002.
- Adam Ismail; Gerard Bannenberg; Harry B. Rice; Ellen Schutt; Douglas Mackay; Oxidation in EPA‐ and DHA‐rich oils: an overview. Lipid Technology 2016, 28, 55-59, 10.1002/lite.201600013.
- Jeroen Maes; Bruno De Meulenaer; Peter Van Heerswynghels; Wim De Greyt; Gauthier Eppe; Edwin De Pauw; André Huyghebaert; Removal of dioxins and PCB from fish oil by activated carbon and its influence on the nutritional quality of the oil. Journal of the American Oil Chemists' Society 2005, 82, 593-597, 10.1007/s11746-005-1114-1.
- M.I. Castro-González; M. Méndez-Armenta; Heavy metals: Implications associated to fish consumption. Environmental Toxicology and Pharmacology 2008, 26, 263-271, 10.1016/j.etap.2008.06.001.
- Matthew R. Miller; Peter D. Nichols; Chris G. Carter; n-3 Oil sources for use in aquaculture – alternatives to the unsustainable harvest of wild fish. Nutrition Research Reviews 2008, 21, 85-96, 10.1017/s0954422408102414.
- Kaspars Ivanovs; Dagnija Blumberga; Extraction of fish oil using green extraction methods: a short review. Energy Procedia 2017, 128, 477-483, 10.1016/j.egypro.2017.09.033.
- Ramakrishnan Vv Ghaly Ae; Ghaly Ae Ramakrishnan Vv; Extraction of Oil from Mackerel Fish Processing Waste using Alcalase Enzyme. Enzyme Engineering 2013, 2, 1-9, 10.4172/2329-6674.1000115.
- Nuria Rubio-Rodríguez; Sagrario Beltrán; Isabel Jaime; Sara M. De Diego; María Teresa Sanz; Jordi Rovira Carballido; Production of omega-3 polyunsaturated fatty acid concentrates: A review. Innovative Food Science & Emerging Technologies 2010, 11, 1-12, 10.1016/j.ifset.2009.10.006.
- Rodrigo Melgosa; María Teresa Sanz; Sagrario Beltrán; Supercritical CO2 processing of omega-3 polyunsaturated fatty acids – Towards a biorefinery for fish waste valorization. The Journal of Supercritical Fluids 2021, 169, 105121, 10.1016/j.supflu.2020.105121.
- M. Alkio; C. Gonzalez; M. Jäntti; O. Aaltonen; Purification of polyunsaturated fatty acid esters from tuna oil with supercritical fluid chromatography. Journal of the American Oil Chemists' Society 2000, 77, 315-321, 10.1007/s11746-000-0051-3.
- Juliane Neubronner; Jan Philipp Schuchardt; Gaby Kressel; Martin Merkel; Clemens Von Schacky; Andreas Hahn; Enhanced increase of omega-3 index in response to long-term n-3 fatty acid supplementation from triacylglycerides versus ethyl esters. European Journal of Clinical Nutrition 2010, 65, 247-254, 10.1038/ejcn.2010.239.
- Cristina Prieto; Lourdes Calvo; The encapsulation of low viscosity omega-3 rich fish oil in polycaprolactone by supercritical fluid extraction of emulsions. The Journal of Supercritical Fluids 2017, 128, 227-234, 10.1016/j.supflu.2017.06.003.
- Soon Hong Soh; Lai Yeng Lee; Microencapsulation and Nanoencapsulation Using Supercritical Fluid (SCF) Techniques. Pharmaceutics 2019, 11, 21, 10.3390/pharmaceutics11010021.
- Fahim Tamzeedul Karim; Kashif Ghafoor; Sahena Ferdosh; Fahad Al-Juhaimi; Eaqub Ali; Kamaruzzaman Bin Yunus; Mir Hoseini Hamed; Ashraful Islam; Mohammad Asif; Mohammed Zaidul Islam Sarker; et al. Microencapsulation of fish oil using supercritical antisolvent process. Journal of Food and Drug Analysis 2017, 25, 654-666, 10.1016/j.jfda.2016.11.017.
- Pratibha Kaushik; Kim Dowling; Colin J. Barrow; Benu Adhikari; Microencapsulation of omega-3 fatty acids: A review of microencapsulation and characterization methods. Journal of Functional Foods 2015, 19, 868-881, 10.1016/j.jff.2014.06.029.
- Atul Dhiman; Pramod K. Prabhakar; Micronization in food processing: A comprehensive review of mechanistic approach, physicochemical, functional properties and self-stability of micronized food materials. Journal of Food Engineering 2021, 292, 110248, 10.1016/j.jfoodeng.2020.110248.
- Atul Dhiman; Pramod K. Prabhakar; Micronization in food processing: A comprehensive review of mechanistic approach, physicochemical, functional properties and self-stability of micronized food materials. Journal of Food Engineering 2021, 292, 110248, 10.1016/j.jfoodeng.2020.110248.
- Atul Dhiman; Pramod K. Prabhakar; Micronization in food processing: A comprehensive review of mechanistic approach, physicochemical, functional properties and self-stability of micronized food materials. Journal of Food Engineering 2021, 292, 110248, 10.1016/j.jfoodeng.2020.110248.
- Jeroen Maes; Bruno De Meulenaer; Peter Van Heerswynghels; Wim De Greyt; Gauthier Eppe; Edwin De Pauw; André Huyghebaert; Removal of dioxins and PCB from fish oil by activated carbon and its influence on the nutritional quality of the oil. Journal of the American Oil Chemists' Society 2005, 82, 593-597, 10.1007/s11746-005-1114-1.
- Matthew R. Miller; Peter D. Nichols; Chris G. Carter; n-3 Oil sources for use in aquaculture – alternatives to the unsustainable harvest of wild fish. Nutrition Research Reviews 2008, 21, 85-96, 10.1017/s0954422408102414.
