Fugitive dust is a serious threat to unpaved road users from a safety and health point of view. Dust suppressing materials or dust suppressants are often employed to lower the fugitive dust. Currently, many dust suppressants are commercially available and are being developed for various applications.
1. Introduction
In the United States, unpaved or gravel roads constitute about 33.1% of the complete road network [1]. A significant portion of these unpaved roads serve as a connection between rural farming communities and urban areas, and the rest of them facilitate pathways to forests, mining fields, and timber hauls [2]. On unpaved roads, fugitive dust emanates from the mechanical interaction between the moving vehicles and the crushed aggregates [3]. Fugitive dust primarily comprises soil minerals (e.g., oxides of silicon, aluminum, calcium, and iron) with particulate material sizes lower than 10 μm (PM 10
) [4]. According to the National Transportation Statistics (NTS) report [1], approximately 18.5 million short tons of PM 10
and 5.34 million short tons of PM
2.5
particulates (size lower than 2.5 μm) are entrained into the air annually. About 35% of this particulate material comes from unpaved roads [5]. From the health, economic, and safety points of view, the generation of fugitive dust poses a serious threat to road users and people living in the vicinity of unpaved roads.
The presence of PM
10
and PM
2.5 in the fugitive dust is found to significantly impact the health of the public, livestock, vegetation, and aquatic life in the premises of unpaved roads by promoting the transport of allergens, spores, and microorganisms [6][7]. While some researchers reported a positive association between PM
in the fugitive dust is found to significantly impact the health of the public, livestock, vegetation, and aquatic life in the premises of unpaved roads by promoting the transport of allergens, spores, and microorganisms [6,7]. While some researchers reported a positive association between PM 2.5 particulates and the cardiovascular and respiratory complications [8][9][10][11][12][13][14], other researchers reported a positive association between PM
particulates and the cardiovascular and respiratory complications [8,9,10,11,12,13,14], other researchers reported a positive association between PM 10 and higher rates of hospitalization due to ischemic heart disease and carcinoma [15][16][17]. A more detailed literature review on various chronic diseases resulting from the fugitive dust can be found elsewhere [18][19][20][21]. In a recent data analysis carried out by Wu et al. [22] using data from the United States, a positive association between the long-term exposure to PM
and higher rates of hospitalization due to ischemic heart disease and carcinoma [15,16,17]. A more detailed literature review on various chronic diseases resulting from the fugitive dust can be found elsewhere [18,19,20,21]. In a recent data analysis carried out by Wu et al. [22] using data from the United States, a positive association between the long-term exposure to PM 2.5
and the increased risk of COVID-19 (Coronavirus disease 2019) was reported, i.e., an increase of only 1 μg/m
3
in PM
2.5
is associated with an 8% increase in the COVID-19 death rate in the United States. In the case of children and elderly people, the finer dust particulates were noticed to aggravate heart and lung diseases such as bronchitis, pneumonitis, wheezing, cardiac artery disease, and cardiac arrhythmias, which can increase the risk of death [23].
When spread in air in higher concentrations, fugitive dust not only adversely impacts the air quality but also obscures the road visibility, leading to the increased risk of accidents, fatalities, and disruption of smooth flow of traffic [4][24][25]. The rate of fatalities on unpaved rural roads in the United States was reported to be more than double when compared to paved urban roads, i.e., for 100 million vehicle miles traveled, the rate of fatalities on unpaved rural roads is 1.8, and the rate of fatalities on paved rural roads is 0.7 [1][26]. The probability of wind-related accidents was determined to contribute to low visibilities, indicating fugitive dust as one of the possible reasons for this increased accident rate on unpaved roads [6]. Examples of some accidents that occurred in the past due to fugitive dust include a chain of vehicle crashes on I-39 Wisconsin [27]; accidents on Interstate 5 in Coalinga, California; a fatal ATV rollover crash in Carlton country, Minnesota [28]; crashes in the intersection of Conejo Avenue and Highway 41, California [29]; crashes on U.S. Highway 87 between Great Falls and Fort Benton [30]; and accidents in Butler County, Missouri [30]. Numerous individual car crashes and mortalities were also recorded in the past on unpaved roads due to the low visibility and dust storms.
When spread in air in higher concentrations, fugitive dust not only adversely impacts the air quality but also obscures the road visibility, leading to the increased risk of accidents, fatalities, and disruption of smooth flow of traffic [4,24,25]. The rate of fatalities on unpaved rural roads in the United States was reported to be more than double when compared to paved urban roads, i.e., for 100 million vehicle miles traveled, the rate of fatalities on unpaved rural roads is 1.8, and the rate of fatalities on paved rural roads is 0.7 [1,26]. The probability of wind-related accidents was determined to contribute to low visibilities, indicating fugitive dust as one of the possible reasons for this increased accident rate on unpaved roads [6]. Examples of some accidents that occurred in the past due to fugitive dust include a chain of vehicle crashes on I-39 Wisconsin [27]; accidents on Interstate 5 in Coalinga, California; a fatal ATV rollover crash in Carlton country, Minnesota [28]; crashes in the intersection of Conejo Avenue and Highway 41, California [29]; crashes on U.S. Highway 87 between Great Falls and Fort Benton [30]; and accidents in Butler County, Missouri [30]. Numerous individual car crashes and mortalities were also recorded in the past on unpaved roads due to the low visibility and dust storms.
Keeping in view the dreadful impacts of fugitive dust on human health and safety, state departments of transportation (DoTs) and local (county/city/rural) agencies often employ maintenance techniques on these unpaved roads, such as paving, blading, speed control, and chemical stabilization to circumvent the entrainment of fugitive dust and to ensure the safety of unpaved road users [24]. Among these techniques, dust suppressants or chemical stabilizers are most widely adopted in practice due to their ease of application and low cost. The commonly employed dust suppressants to control the fugitive dust include water, calcium chloride (CaCl 2
), magnesium chloride (MgCl
2), and other chloride salts. However, the performances of the dust suppressants vary depending on their physical and chemical characteristics, application rates, soil type, wind speed, atmospheric conditions, etc. Presently, there are more than 200 dust-suppressing products available on the market [31].
), and other chloride salts. However, the performances of the dust suppressants vary depending on their physical and chemical characteristics, application rates, soil type, wind speed, atmospheric conditions, etc. Presently, there are more than 200 dust-suppressing products available on the market [31].
To the best of our knowledge, a comprehensive review of contemporary research into the dust-suppressing materials and their working mechanisms is not available in the literature. While it is necessary to understand the characteristics and the working mechanism of dust-suppressing materials from the field application perspective, it is also important to be informed about the process involved in synthesizing the material briefly that would be of interest for many practicing environmental, structural, and transportation engineers. The current review paper not only focuses on describing various working mechanisms involved in suppressing dust using dust suppressants but also provides a brief overview of the process involved in their synthesis. The rest of the manuscript is organized as follows. Description of the dust suppression mechanisms is provided in Section 2; a review of various categories of dust suppressants, their synthesis, and advantages are described in Section 3; and the highlights from the review and the recommendations are provided in Section 4.
2. Dust Suppression Mechanisms
The efficiency of dust suppressants is based on one or both of two underlying mechanisms, namely, hygroscopicity and agglomeration. In this section, a brief description of these two mechanisms is provided.
2.1. Hygroscopicity
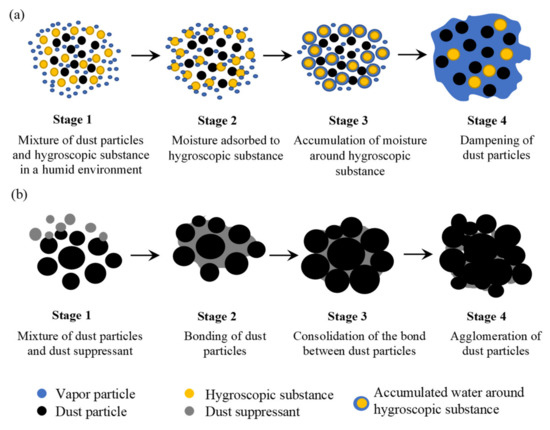
Hygroscopicity refers to the ability of a solid substance to absorb or adsorb moisture from the surrounding atmosphere [32][33]. Owing to their affinity to water, hygroscopic substances can retain moisture and maintain a dampened, hard, and compact road surface, which subsequently prevents the erosion of fugitive dust. On the basis of the mechanism of water absorption, one can categorize the hygroscopic materials into two classes, namely, chemical and physical hygroscopic materials [34][35][36]. While chemical hygroscopic materials absorb water via a chemical reaction that converts their entire nature (e.g., metal hydrides), the physical hygroscopic materials imbibe water vapor through the following four mechanisms: (i) surface adsorption, (ii) condensation in capillaries (e.g., soft polyurethane sponge), (iii) reversible changes of the crystal structure (e.g., silica gel and anhydrous inorganic salt), and (iv) combination of capillary forces and hydration of functional groups (e.g., hydrogels and superabsorbent polymers). The detailed description of these mechanisms can be found elsewhere [37]. Interestingly, a hygroscopic substance may deliquesce if its critical relative humidity (CRH) is lower than that of the surrounding atmosphere, i.e., the water adsorbed on the surface of the hygroscopic substance starts to solvate molecules to an extent that the complete substance is liquified [32][38][39]. Often, deliquescent substances (e.g., CaCl
Hygroscopicity refers to the ability of a solid substance to absorb or adsorb moisture from the surrounding atmosphere [32,33]. Owing to their affinity to water, hygroscopic substances can retain moisture and maintain a dampened, hard, and compact road surface, which subsequently prevents the erosion of fugitive dust. On the basis of the mechanism of water absorption, one can categorize the hygroscopic materials into two classes, namely, chemical and physical hygroscopic materials [34,35,36]. While chemical hygroscopic materials absorb water via a chemical reaction that converts their entire nature (e.g., metal hydrides), the physical hygroscopic materials imbibe water vapor through the following four mechanisms: (i) surface adsorption, (ii) condensation in capillaries (e.g., soft polyurethane sponge), (iii) reversible changes of the crystal structure (e.g., silica gel and anhydrous inorganic salt), and (iv) combination of capillary forces and hydration of functional groups (e.g., hydrogels and superabsorbent polymers). The detailed description of these mechanisms can be found elsewhere [37]. Interestingly, a hygroscopic substance may deliquesce if its critical relative humidity (CRH) is lower than that of the surrounding atmosphere, i.e., the water adsorbed on the surface of the hygroscopic substance starts to solvate molecules to an extent that the complete substance is liquified [32,38,39]. Often, deliquescent substances (e.g., CaCl 2
, MgCl
2
, FeCl
2
etc.) are employed in practice for dust suppression [40]. The mechanism of dust suppression through hygroscopicity involves four stages (see a), namely, Stage 1: the deliquescent dust suppressant is sprayed or mixed with the dust particles; Stage 2: deliquescent substance starts to absorb moisture, and water molecules start to accumulate around the deliquescent substance; Stage 3: the outer layer of the deliquescent substance gets dissolved in the absorbed water; and Stage 4: most of the deliquescent substance gets dissolved in the absorbed water that also contains the dust particle in the formed solution, thereby capturing the dust.
Figure 1.
Schematic of dust suppression mechanisms: (
a
) hygroscopicity and (
b
) agglomeration.
2.2. Agglomeration
Agglomeration-based dust suppression is obtained when binding or cementing agents are introduced into the dust particles. Agglomeration is referred to as the process of converting small diameter solid particles into larger diameter granules that are composed of smaller particles. The binding or cementing agent introduces adhesive forces among the particles to accumulate a larger number and mass of smaller particles. As the mass of agglomerated particles increase, the constituent dust particles are less prone to become airborne. Examples of agglomeration-based dust suppressants include corn starch hydrogels, guar gum, chitosan, different surfactants, and oil-based substances. Similar to hygroscopicity, the mechanism of dust suppression through agglomeration involves four stages (see
b), namely, Stage 1: the dust suppressant is applied on the top layer of the unpaved road; Stage 2: the dust suppressant starts to form an adhesive bridge among the dust particles; Stage 3: the adhesive bridge starts to solidify between the dust particles; and Stage 4: the lump of the agglomerated dust particles grow in size, thus suppressing fugitive dust. However, agglomeration can take place on the top surface only where the agglomerated particles create a protective layer that resists the particles underneath it to become fugitive.