Pain can be induced by tissue injuries, diseases and infections. The interactions between the peripheral nervous system (PNS) and immune system are primary actions in pain sensitizations. In response to stimuli, nociceptors release various mediators from their terminals that potently activate and recruit immune cells, whereas infiltrated immune cells further promote sensitization of nociceptors and the transition from acute to chronic pain by producing cytokines, chemokines, lipid mediators and growth factors. Immune cells not only play roles in pain production but also contribute to PNS repair and pain resolution by secreting anti-inflammatory or analgesic effectors.
- peripheral nervous system
- pain
- immune response
- inflammation
1. Introduction
Pain can be induced by tissue injury or different types of diseases that affect the somatosensory system, resulting in noxious (hyperalgesia) or non-noxious (allodynia) symptoms, which is an important defense mechanism to avoid harmful stimuli. Terminal nerves of somatosensory neurons (also known as nociceptors) innervate into the skin, cornea, internal organs, joints, bones, muscles, and deep visceral tissues, which are highly expressing a set of molecular sensors including transient receptor potential channel subtypes (TRPs), G protein coupled receptors (GPCRs) and sodium channel (Nav) [1][2][1,2]. Upon sensing noxious stimuli (e.g., mechanical, thermal and chemical), these nociceptors can quickly generate action potentials that are transmitted to the central nervous system (CNS) where the signals are processed. Nociceptor sensitization (or peripheral sensitization) at the site of the injury is therefore considered to be the primary cause of pain and the most appropriate targeting system for pain therapies [3][4][3,4].
Pain syndromes can be divided into acute and chronic stages. Acute pain plays a vital protective and adaptive role in warning the individual to avoid further injury and driving immune responses against infections or pathogens during healing. The inflammatory mediators produced by the immune system such as cytokines, lipid mediators, and growth factors directly activate nociceptive primary sensory neurons in the peripheral nervous system (PNS) evoking a pain response [4][5][4,5]. On the other hand, chronic pain is detrimental and arises from nerve damage caused by surgery, trauma, metabolic disorders (e.g., diabetic mellitus) or autoimmune diseases (e.g., multiple sclerosis) [6]. Chronic pain is a long-lasting syndrome and has substantial impacts on patients’ quality of life and high economic burdens on individuals and society [7]. Although alterations in the dorsal spinal cord and brain are one of the key mechanisms of chronic pain maintenance, peripheral sensitization is essential in the transition from the acute to the chronic stage [5][8][5,8]. Notably, emerging studies have revealed that bidirectional signaling between the immune and nervous systems contribute to the initiation and maintenance of chronic pain [2]. Altogether, our current knowledge of nociceptor–immune interactions have provided some molecular insights for developing better therapies for pathological inflammation-associated pain.
2. Peripheral Responses to Pain
Like the counterparts in the CNS, the PNS is also composed of neurons and glial cells, in which clusters of nociceptive sensory neurons are located in different ganglion in the trunk and the head that relay information about the environment to the CNS. The most common types of ganglion are the dorsal root ganglion (DRG) in the trunk, and others in the cranial including trigeminal and glossopharyngeal ganglia [9]. These sensory neurons (first-order primary afferent neurons) are classified to unmyelinated c-fibers and myelinated Aδ/β -fibers that transduce different types of pain signals, including mechanical, thermal, or chemical stimuli (Figure 1a). Free peripheral nerve endings function as receptive sites extend from neuronal cell bodies in the DRG or cranial nerve ganglion. Notably, their sensory neurons are pseudo-unipolar neurons that have one axon with two processes: one peripheral axonal branch innervates the tissues in the body to receive sensory information and the other axonal branch sends nerve impulses to excite second-order postsynaptic neurons in the dorsal horn of the spinal cord [3][5][3,5]. Subsequently, axons from second-order neurons project into thalamic nuclei in brain, where the third-order neurons transmit the pain signaling to the primary sensory cortex [10] (Figure 1b). The glial cells in the PNS mainly comprise Schwann cells and satellite glial cells (SGCs). The SGCs surround the somata of sensory neurons and usually consist of a single layer of cells connected to each other by gap junctions [11]. Schwann cells are the most abundant cell types in the PNS, which support axonal outgrowth by producing a variety of growth factors, such as nerve growth factor (NGF), glial cell line derived neurotrophic factor (GDNF), and brain-derived neurotrophic factor (BDNF) [12][13][12,13]. Schwann cells consist of two major phenotypes, myelinating Schwann cells and nonmyelinating Schwann cells [12]. Myelinating Schwann cells wrap larger axons in a 1:1 ratio to form the myelin sheath, nonmyelinating Schwann cells embed smaller axons, forming a remark bundle [13] (Figure 1c).
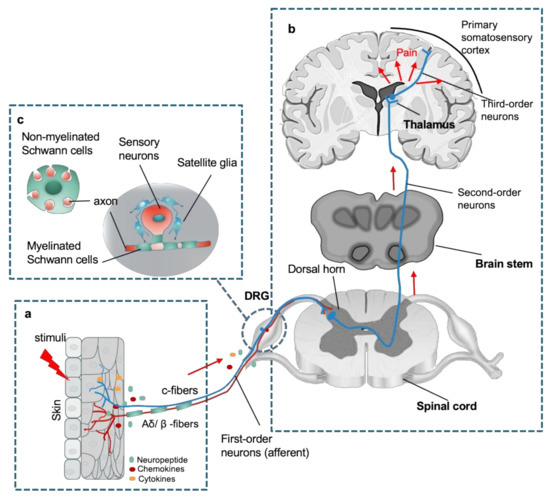
Figure 1.
a
b
c
b
After noxious stimuli, peripheral neurons/nerves and glial cells undergo significant pathological changes before central properties that contribute to the pain initiation and development through their interaction with immune signals. Noteworthy, signaling pathways between primary sensory neurons, SGCs, Schwann cells and immune cells are highly intertwined. For example, activated Schwann cells mediate the breakdown of the blood–nerve barrier via the secretion of matrix metalloproteinase 9 (MMP-9), which promotes the recruitment and infiltration of immune cells (e.g., macrophages and T cells) from the vasculature to the injury sites. [14][15][14,15]. Sensory neurons also produce neuropeptides at their peripheral endings that not only serve as attractions to induce the invasion of circulating immune cells but also modulate the activity of innate and adaptive immune cells [2][16][2,16]. A dense cluster of immune cells produce pronociceptive mediators directly acting on peripheral nociceptors to promote sensitization of pain signaling and the recruitment of immune cells and vice versa. In addition, reactive SGCs and immune cells (e.g., macrophages) work cooperatively to promote peripheral sensitization by releasing proinflammatory cytokines such as IL-1β, IL-6, and TNF [17][18][19][20][21][17,18,19,20,21]. In contrast, SGCs and macrophages are also involved in the regeneration of DRG axons and remyelination of Schwann cells [21][22][21,22]. The four major heterogeneous immune cell types (macrophages and/or monocytes, mast cells, neutrophils, and T cells) resident in or infiltrating the PNS have specific functions in pain modulation and sensitization.
