Galectin-3 is a member of the galectins family of carbohydrate-binding proteins with specificity for N-acetyllactosamine (LacNAc)-containing glycoproteins, and the only known one with a single carbohydrate recognition domain and a unique N-terminus.
Definition:
Galectin-3 is a member of the galectins family of carbohydrate-binding proteins with specificity for N-acetyllactosamine (LacNAc)-containing glycoproteins, and the only known one with a single carbohydrate recognition domain and a unique N-terminus.
- extracorporeal life support
- mechanical circulatory support
- ECMO
- VAD
- galectin-3
1. Introduction:
Galectin-3 is a member of the galectins family of carbohydrate-binding proteins with specificity for N-acetyllactosamine (LacNAc)-containing glycoproteins, and the only known one with a single carbohydrate recognition domain and a unique N-terminus [1][2]. It is a 30 kDa molecule encoded by the LGALS3 gene that is located on chromosome 14, locus q21–q22 [3]. It is mainly secreted by macrophages and regulates basic cellular functions including growth, proliferation, differentiation and inflammation [4][5][6][7] and importantly has been found to play a role in cardiac fibrosis [8][9]. Studies have suggested that galectin-3 can help to predict prognosis of heart failure and adverse events in various clinical settings such as patients with ST elevation myocardial infraction [10], congenital heart disease patients with a Fontan circulation [11] and survivors of out-of-hospital cardiac arrest [12]. In addition, its levels have been correlated with morbidity and mortality in patients with heart failure [13][14][15][16].
Galectin-3 is a member of the galectins family of carbohydrate-binding proteins with specificity for N-acetyllactosamine (LacNAc)-containing glycoproteins, and the only known one with a single carbohydrate recognition domain and a unique N-terminus [1,2]. It is a 30 kDa molecule encoded by the LGALS3 gene that is located on chromosome 14, locus q21–q22 [3]. It is mainly secreted by macrophages and regulates basic cellular functions including growth, proliferation, differentiation and inflammation [4,5,,6,7] and importantly has been found to play a role in cardiac fibrosis [8,9]. Studies have suggested that galectin-3 can help to predict prognosis of heart failure and adverse events in various clinical settings such as patients with ST elevation myocardial infraction [10], congenital heart disease patients with a Fontan circulation [11] and survivors of out-of-hospital cardiac arrest [12]. In addition, its levels have been correlated with morbidity and mortality in patients with heart failure [13,14,15,16].
2. Possible mechanism of galectin 3 in heart failure
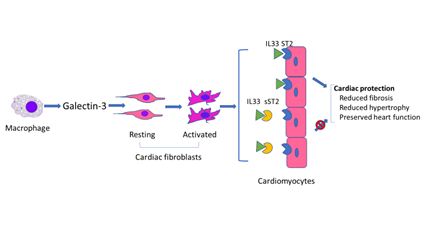
In response to cardiac injury, activated macrophages produce galectin-3 which is then thought to regulate phenotypic change of cardiac fibroblasts from the resting to the activated status [17], whereas sST2 binds to IL33 to block the binding of IL33 to ST2 on cardiomyocytes. Binding of IL33 to cardiomyocyte membrane ST2 results in the initiation of IL33/ST2 pathway which then evokes an antihypertrophic and antifibrotic function [18].
Schematic of possible mechanism of galectin 3 in heart failure
3. Application of galectin-3 in adult and pediatric patients with heart failure requiring ECLS
Application of galectin-3 in adult and pediatric patients with heart failure requiring ECLS
Heart failure is a life-threatening condition in both adults and children and is associated with high mortality, morbidity, and cost of care. Extracorporeal life support (ECLS) including ventricular assist device (VAD) implantation and extracorporeal membrane oxygenation (ECMO) is required for patients with advanced or end-staged heart failure either as destination therapy or as a bridge-to-transplantation therapy. Galectin-3 is one of biomarkers that has been employed in attempt to predict these outcomes in adult and pediatric patients with heart failure requiring ECLS, but there is significantly less literature regarding galectin-3 in pediatric patients as compared to adults, as shown in Table below. Higher values (>15.3 ng/mL) of galectin-3 have been reported to show a correlation with the severity of heart failure [19].
|
Reference |
Year |
Adult/Peds |
N = |
Population |
Major Finding |
|
[20]20 |
2008 |
Adult |
40 |
VAD |
Higher Gal-3 pre implant associated with mortality (n=15) compared to bridged to transplant(n=25) (13.4 + 3.6 ng/ml vs. (9.6 + 5.2 ng/ml, p<0.02) |
|
[21]21 |
2013 |
Adult |
175 |
VAD |
Higher Galectin-3 levels (>17ng/ml) increased mortality for low/medium risk VAD patients |
|
[22]22 |
2015 |
Adult |
25 |
VAD |
Gal-3 remains elevated after continuous flow VAD placed. |
|
[23]23 |
2015 |
Adult |
37 |
VAD |
Gal-3 decreases during LVAD support |
|
[19]24 |
2016 |
Adult |
57 |
VAD |
Galectin-3 levels >30 ng/ml are associated with lower survival post-LVAD placement (76.5 % versus 95.0 % at 2 years, p = 0.009 |
|
[24]25 |
2018 |
Both |
7 adult /12 pediatric |
VAD |
Children similar Galectin-3 levels as adults post VAD |
Perspective
Extracorporeal life support (ECLS) including ventricular assist device (VAD) implantation and extracorporeal membrane oxygenation (ECMO) is required for patients with advanced or end-staged heart failure either as destination therapy or as a bridge-to-transplantation. Undergoing ECLS creates a complex clinical situation with challenges related to early and accurate prediction of prognosis, particularly in pediatric patients. To distinguish patients, who will improve and those who will not early during ECLS, is imperative as it would not only assist the medical team to formulate an optimal care plan but may also provide a scientific justification to initiate ethical discussions with the patient’s family. Galectin 3 along with other biomarkers, for instance, sST2, have been documented as prognostic markers for myocardial recovery in patients with refractory heart failure requiring circulatory support. However, neither galectin-3 and/or sST2 has been examined as guides for adjusting medical management for heart failure in pediatric patients, and thus the role of galectin-3 and /or sST2 as a guide to therapeutic decision-making remains to be established.
4. Perspective
References
Extracorporeal life support (ECLS) including ventricular assist device (VAD) implantation and extracorporeal membrane oxygenation (ECMO) is required for patients with advanced or end-staged heart failure either as destination therapy or as a bridge-to-transplantation. Undergoing ECLS creates a complex clinical situation with challenges related to early and accurate prediction of prognosis, particularly in pediatric patients. To distinguish patients, who will improve and those who will not early during ECLS, is imperative as it would not only assist the medical team to formulate an optimal care plan but may also provide a scientific justification to initiate ethical discussions with the patient’s family. Galectin 3 along with other biomarkers, for instance, sST2, have been documented as prognostic markers for myocardial recovery in patients with refractory heart failure requiring circulatory support. However, neither galectin-3 and/or sST2 has been examined as guides for adjusting medical management for heart failure in pediatric patients, and thus the role of galectin-3 and /or sST2 as a guide to therapeutic decision-making remains to be established.
- Elola, M.T.; Wolfenstein-Todel, C.; Troncoso, M.F.; Vasta, G.R.; Rabinovich, G.A. Galectins: Matricellular glycan-binding proteins linking cell adhesion, migration, and survival. Cell. Mol. Life Sci. 2007, 64, 1679–1700.
- Hughes, R.C. The galectin family of mammalian carbohydrate-binding molecules. Biochem. Soc. Trans. 1997, 25, 1194–1198.
- Meijers, W.C.; Van Der Velde, A.R.; De Boer, R.A. The ARCHITECT galectin-3 assay: Comparison with other automated and manual assays for the measurement of circulating galectin-3 levels in heart failure. Expert Rev. Mol. Diagn. 2014, 14, 257–266.
- Papaspyridonos, M.; McNeill, E.; de Bono, J.P.; Smith, A.; Burnand, K.G.; Channon, K.M.; Greaves, D.R. Galectin-3 is an amplifier of inflammation in atherosclerotic plaque progression through mac-rophage activation and monocyte chemoattraction. Arterioscler. Thromb. Vasc. Biol 2008, 28, 433–440.
- Zhuo, Y.; Chammas, R.; Bellis, S.L. Sialylation of beta1 integrins blocks cell adhesion to galectin-3 and protects cells against galectin-3-induced apoptosis. J. Biol. Chem. 2008, 283, 22177–22185.
- Henderson, N.C.; MacKinnon, A.C.; Farnworth, S.L.; Kipari, T.; Haslett, C.; Iredale, J.P.; Liu, F.-T.; Hughes, J.; Sethi, T. Galectin-3 Expression and Secretion Links Macrophages to the Promotion of Renal Fibrosis. Am. J. Pathol. 2008, 172, 288–298.
- Yang, E.; Shim, J.S.; Woo, H.-J.; Kim, K.-W.; Kwon, H.J. Aminopeptidase N/CD13 induces angiogenesis through interaction with a pro-angiogenic protein, galectin-3. Biochem. Biophys. Res. Commun. 2007, 363, 336–341.
- Martínez-Martínez, E.; Brugnolaro, C.; Ibarrola, J.; Ravassa, S.; Buonafine, M.; López, B.; Fernández-Celis, A.; Querejeta, R.; Santamaria, E.; Fernández-Irigoyen, J.; et al. CT-1 (Cardiotrophin-1)-Gal-3 (Galectin-3) Axis in Cardiac Fibrosis and Inflammation. Hypertension 2019, 73, 602–611.
- Martínez-Martínez, E.; Ibarrola, J.; Fernández-Celis, A.; Santamaria, E.; Fernández-Irigoyen, J.; Rossignol, P.; Jaisser, F.; LopezAndres, N. Differential Proteomics Identifies Reticulocalbin-3 as a Novel Negative Mediator of Collagen Production in Human Cardiac Fibroblasts. Sci. Rep. 2017, 7, 1–10.
- Tyminska, A.; Kapłon-Cie´slicka, A.; Oziera ´nski, K.; Budnik, M.; Wancerz, A.; Sypie ´n, P.; Peller, M.; Balsam, P.; Opolski, G.; Filipiak, K.J. Association of Galectin-3 and Soluble ST2, and Their Changes, with Echocardiographic Parameters and Development of Heart Failure after ST-Segment Elevation Myocardial Infarction. Dis. Markers 2019, 2019, 9529053.
- Opotowsky, A.; Baraona, F.; Owumi, J.; Loukas, B.; Singh, M.N.; Valente, A.M.; Wu, F.; Cheng, S.; Veldtman, G.; Rimm, E.B.; et al. Galectin-3 Is Elevated and Associated with Adverse Outcomes in Patients with Single-Ventricle Fontan Circulation. J. Am. Hear. Assoc. 2016, 5, e002706.
- Mosleh, W.; Kattel, S.; Bhatt, H.; Al-Jebaje, Z.; Khan, S.; Shah, T.; Dahal, S.; Khalil, C.; Frodey, K.; Elibol, J.; et al. Galectin-3 as a Risk Predictor of Mortality in Survivors of Out-of-Hospital Cardiac Arrest. Circ. Arrhythmia Electrophysiol. 2019, 12, e007519.
- Van Kimmenade, R.R.; Januzzi, J.L., Jr.; Ellinor, P.T.; Sharma, U.C.; Bakker, J.A.; Low, A.F.; Martinez, A.; Crijns, H.J.; MacRae, C.A.; Menheere, P.P.; et al. Utility of amino-terminal pro-brain natriuretic peptide, galectin-3, and apelin for the evaluation of patients with acute heart failure. J. Am. Coll. Cardiol. 2006, 48, 1217–1224.
- Sygitowicz, G.; Tomaniak, M.; Filipiak, K.J.; Kołtowski, Ł.; Sitkiewicz, D. Galectin-3 in Patients with Acute Heart Failure: Preliminary Report on First Polish Experience. Adv. Clin. Exp. Med. 2016, 25, 617–623.
- George, M.; Shanmugam, E.; Srivatsan, V.; Vasanth, K.; Ramraj, B.; Rajaram, M.; Jena, A.; Sridhar, A.; Chaudhury, M.; Kaliappan, I. Value of pentraxin-3 and galectin-3 in acute coronary syndrome: A short-term prospective cohort study. Ther. Adv. Cardiovasc. Dis. 2015, 9, 275–284.
- McEvoy, J.W.; Chen, Y.; Halushka, M.K.; Christenson, E.; Ballantyne, C.M.; Blumenthal, R.S.; Christenson, R.H.; Selvin, E. Galectin-3 and Risk of Heart Failure and Death in Blacks and Whites. J. Am. Hear Assoc. 2016, 5, e003079.
- De Boer, R.A.; Voors, A.A.; Muntendam, P.; van Gilst, W.H.; van Veldhuisen, D.J. Galectin-3: A novel mediator of heart failure development and progression. Eur. J. Heart Fail. 2009, 11, 811–817.
- Chow, S.L.; Maisel, A.S.; Anand, I.; Bozkurt, B.; De Boer, R.A.; Felker, G.M.; Fonarow, G.C.; Greenberg, B.; Januzzi, J.L.; Kiernan, M.S.; et al. Role of Biomarkers for the Prevention, Assessment, and Management of Heart Failure: A Scientific Statement from the American Heart Association. Circulation 2017, 135, e1054–e1091.
- Ueland, T.; Aukrust, P.; Broch, K.; Aakhus, S.; Skårdal, R.; Muntendam, P.; Gullestad, L. Galectin-3 in heart failure: High levels are associated with all-cause mortality. Int. J. Cardiol. 2011, 150, 361–364.
- Emdin, M.; Aimo, A.; Vergaro, G.; Bayes-Genis, A.; Lupón, J.; Latini, R.; Meessen, J.; Anand, I.S.; Cohn, J.N.; Gravning, J.; et al. sST2 Predicts Outcome in Chronic Heart Failure Beyond NT-proBNP and High-Sensitivity Troponin T. J. Am. Coll. Cardiol. 2018, 72, 2309–2320.
- Coromilas, E.; Que-Xu, E.-C.; Moore, D.; Kato, T.S.; Wu, C.; Ji, R.; Givens, R.; Jorde, U.P.; Takayama, H.; Naka, Y.; et al. Dynamics and prognostic role of galectin-3 in patients with advanced heart failure, during left ventricular assist device support and following heart transplantation. BMC Cardiovasc. Disord. 2016, 16, 1–10.
- Lok, S.I.; Nous, F.M.; Van Kuik, J.; Van Der Weide, P.; Winkens, B.; Kemperman, H.; Huisman, A.; Lahpor, J.R.; De Weger, R.A.; De Jonge, N. Myocardial fibrosis and pro-fibrotic markers in end-stage heart failure patients during continuous-flow left ventricular assist device support. Eur. J. Cardiothorac. Surg. 2015, 48, 407–415.
- Ahmad, T.; Wang, T.; O’Brien, E.C.; Samsky, M.D.; Pura, J.A.; Lokhnygina, Y.; Rogers, J.G.; Hernandez, A.F.; Craig, D.; Bowles, D.E.; et al. Effects of Left Ventricular Assist Device Support on Biomarkers of Cardiovascular Stress, Fibrosis, Fluid Homeostasis, inflammation, and Renal Injury. JACC Hear Fail. 2015, 3, 30–39.
- Ueland, T.; Aukrust, P.; Broch, K.; Aakhus, S.; Skårdal, R.; Muntendam, P.; Gullestad, L. Galectin-3 in heart failure: High levels are associated with all-cause mortality. Int. J. Cardiol. 2011, 150, 361–364.
- Kramer, F.; Milting, H. Novel biomarkers in human terminal heart failure and under mechanical circulatory support. Biomarkers 2011, 16, S31–S41.