Central serous chorioretinopathy (CSC) is a controversial disease both in terms of clinical classification and choice of therapeutic strategy. Choroidal layers, retinal pigment epithelium (RPE), photoreceptors, and retina are involved to varying degrees.
- central serous chorioretinopathy
- fluorescein angiography
- optical coherence topography
1. Introduction
In many ways, central serous chorioretinopathy (CSC) still represents a somewhat mysterious disease. There is mounting scientific evidence that a combination of malfunctioning choroid (thick and hyperpermeable) and damaged retinal pigment epithelium (RPE) is the basis for this disease. In the past, imaging in CSC has been represented mainly by fluorescein angiography (FA) and indocyanine green angiography (ICGA). Newer techniques such as fundus autofluorescence (FAF), optical coherence tomography (OCT), and OCT angiography (OCT-A) have been added, enriching immensely our knowledge about mechanisms that originate CSC. Beyond a role in differential diagnosis with other potentially confounding diseases, multimodal imaging allows to plan the best treatment according to the CSC stage and the degree of choroid, photoreceptors, and RPE involvement.
The main clinical sign is the appearance of subretinal fluid (SRF), leaked into the subretinal space through one or multiple RPE defects. An example of multimodal imaging in an eye with acute CSC is visible in Figure 1.
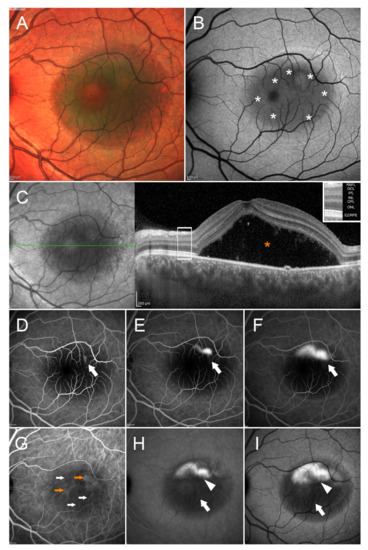
Figure 1.
A
B
C
D
E
F
G
H
I
2. OCT Features in CSC Eyes
Pachychoroid
In all likelihood, CSC is a clinical entity belonging at least in part to the pachychoroid spectrum, a phenotype characterized by rarefaction of choriocapillaris with overlying dilated choroidal veins on EDI-OCT, as well as with progressive RPE dysfunction and increased risk of CNV [1][2][3][4]. Pachychoroid (choroidal thickness >270 μm on EDI-OCT and/or presence of pachyvessels) is secondary to enlargement of Haller’s layer and subsequent compression on inner choroid vessels [5][6][7]. Other diseases, including pachychoroid pigment epitheliopathy (PPE), pachychoroid neovasculopathy (PCN) and PCV may manifest with pachychoroid [6][7]. Nevertheless, a thicker choroid is not a mandatory diagnostic criterion for CSC.
En face SS-OCT identified anomalies as thinning of the inner choroidal layer in the involved area (maybe due to a choriocapillaris atrophy or to compression exerted by dilated outer choroidal vessels), [1] focal or diffuse dilation of various degree, involving one or more layers [3] or areas of choroidal thickening topographically associated with pathologically dilated veins of the Haller’s layer (or “pachyvessels”), [2] abnormal hyperreflective areas involving Bruch’s membrane and choriocapillaris, corresponding to hypofluorescent areas seen on ICGA late phases [4]. As already stated above, a thicker choroid at baseline is a positive predictor for a better response to treatment with eplerenone [8].
Hyperreflective Dots (HRD)
In active chronic CSC, the presence of HRDs in inner choroid as well as hyperreflectivity of dilated vessels wall has been documented [9]. HRDs may appear also in SRF and increase in number as the disease duration increases. It is not precisely known at what HRDs do correspond, but they may be the equivalent of photoreceptor outer segments shedding, activated microglia and macrophages, or concentrated fibrin or lipid compounds. A recent study about HRDs reaffirmed that they are not a peculiar sign of CSC, since they have been described in macular dystrophies, age-related macular degeneration (AMD), retinal vein occlusion and diabetic retinopathy [10]. Authors found that in acute CSC, HRDs presence is correlated with subfoveal choroidal thickness and patients age, whereas in chronic CSC they are associated with macular and choroidal thickness as well the height of neurosensory detachment. In this latter group of eyes, HRDs could indicate an ongoing process of anatomical rearrangement of the choroid or phagocytosis of photoreceptors outer segments. Therefore, the higher the baseline HRDs number on SD-OCT, the higher the chances of recurrence after treatment [11]. Another report described that HRDs and subretinal exudates are more commonly observed in early- and late-chronic CSC than in acute CSC [12].
Posterior Cystoid Retinal Degeneration (PCRD)
In longlasting chronic CSC, SD-OCT may identify PCRD. It is a severe disease phenotype characterized by DARA, multiple leaking spots on FA, subretinal fibrin deposits at first presentation, [13][14][15][16][17] so creating a distinct entity within CSC, one with unfavourable visual prognosis [14]. PCRD was shown to be present in up to 35% of severe chronic CSC cases [14]. In contrast to cystoid macular edema (cystic fluid within Henle layer), PCRD may have a degenerative origin, descending from the primary choroidopathy and the RPE dysfunction, and do not stain on FA [17]. Apparently, few or no VEGF-related pathway appear involved in PCRD onset (anti-VEGF drugs are mostly ineffective in these eyes) [18]. Not rarely there is some active SRF leakage and RPE function is irreversibly damaged [19]. Treatment may help in halting some SRF leakage, but is less likely to result in a complete PCRD resolution and/or a BCVA improvement [19], since foveal damage and vision loss may be secondary to intraretinal fluid itself, as well as an associated foveal detachment [20]. In a recent report, OCT-A detected a CNV in 13 out of 29 eyes with PCRD [21].
Pigment Epithelial Detachment (PED)
In acute CSC a PED is identified by OCT in 53–100% of eyes [1][22][23] and not rarely colocalizes with the leakage point(s) on FA. Usually it is serous and can be found both within or outside the SRF. Its site corresponds well with areas characterized by vessels abnormalities on EDI-OCT and hyperpermeability on ICGA [1][24]. Another PED pattern identifiable in chronic CSC is defined as shallow or flat irregular PED, divided into vascularized or non-vascularized, with a potentially significant impact on treatment choice [7]. According to Song et al, low-to-flat PED(s) can be observed in all disease stages, particularly in late chronic CSC [12].
The Double Layer Sign (DLS)
The double-layer sign (DLS) is an OCT finding, produced by a shallow irregular PED. The upper hyperreflective band of the double layer belongs to RPE, the bottom band to the Bruch’s membrane. [25]. This finding was first reported in eyes with PCV and later in eyes with AMD and high myopia. It can be found in eyes with CSC, and usually corresponds to thinning of inner choroid and pachyvessels [1][26][27]. Risk of developing secondary CNV increases when a DLS is identifiable and some authors postulated that CSC eyes with DLS may host inactive CNV [28][29]. The space within the DLS could be hypo- or hyperreflective. DLS with internal hyporeflectivity are usually avascular, whereas an internal hyperreflectivity may host early type 1 CNV, even if it is not true in all cases [30]. Differentiating such vascularized from non-vascularized flat PED becomes difficult on conventional imaging. OCT-A has a sensitivity of 86–100% and specificity of 96–100% compared with FA in detecting CNV in eyes with chronic CSC and irregular PED [31][32]. With the advent of OCT-A, the detection of CNV in such eyes has increased significantly (up to 35%) compared with previous imaging modalities. The verified presence of an active CNV may warrant a different therapeutic approach.
Presence of Subretinal Fibrin
Usi
CSC Complicated by CNV
Ing SD-OCT, the presence of SRF can be both assessed and quantified, which is generally considered useful for estimating the episode duration and for determining the subsequent treatment strategy [12]. chronic CSC, OCT can reveal-A fibrin clots within SRF originating from fibrinogen leaked through RPE defect [33]. Abnormalitiemay s (subretinal exudation, retinal dipping, photoreceptor layes defects) were noted by the authors in close proximity to RPE defects in all 123 eyes included in the study. Subretinal fibrin can be observed as both well-defined and ill-defined hyperreflective structures [34]. A severe but unfrequent variant pport ICGA in the detection of chronic CSC is accompanied by bullous retinal detachmentNV. ItFigure 4 is generated by multiple RPE leaks and not rarely subretinal fibrin accumulates in large amount. In some cases it is accompanied by complete disruption of the PED edges, with consequent RPE avulsion (rip or tear) [35]. Another OCT featummarizes how valure, the retinal dragging with fibrin, has been reported, mainly in eyes with acute CSC at its early stages [12].
Liang et al. reble SD-OCT cently reported that in CSC eyes with subretinal fibrin HD-PDT with verteporfin is safe and effective, with no statistically significant difference for SRF and fibrin resolution with respect to eyes without subretinal fibrin at baselinen be in identifying CNV during the course [36].
CSC Complicated by CNV
In chronicf CSC, OCT-A may support ICGA in the detection of CNV.
FA shows disclose a hyperfluorescent alteration with surrounding leakage that on ICGA corresponds mainly to a hypocyanescent anomaly. SD-OCT reveals a hyperreflective subretinal lesion corresponding to CNV, with minimal exudation and intraretinal cysts.
CNV can be found in 2–18% of chronic CSC patients [37][38][39][40][141,142,143,144]. A recent report identified older age, wide PED width at diagnosis and recurrent episodes of CSC as independent risk factors for the development of CNV [41][145]. In addition, the authors found that CSC patients with secondary CNV had lower choriocapillary flow densities than those without secondary CNV. The presence of a PED at initial diagnosis was associated with an approximately 12-fold increased risk of secondary CNV. In addition, it has been suggested that DLS in eyes with CSC usually correspond to flat irregular PED and is associated with the development of CNV [42][146]. The neovascular activity of flat irregular PED at CSC initial phase could be considered of low grade. However, an active form of CNV may appear later, in the chronic phase. Recently, an OCT-A study of chronic CSC revealed that CNV was detected in one-third of eyes with flat irregular PED, which usually corresponded to DLS [29][134].
CNV tends to appear gradually, especially in patients over the age of 50 and/or with longstanding disease (Figure 4). Subretinal leakage from type 1 (sub-RPE) CNV due to pachychoroid neovasculopathy may resemble uncomplicated chronic CSC [7][39][114,143]. CNV can be identified using multimodal imaging techniques such as OCT, FA, ICGA, and—in particular—OCT-A. An example of OCT-A features in a CSC eye with sub-RPE neovascular network is provided in Figure 5.
On FAF there is a mixed pattern of changes (both hypo- and hyperautofluorescent), with an irregular autofluorescence in the lower portion of the posterior pole (gravitational disposition of fluid). A hyperfluorescent lesion with perilesional leakage on FA (mainly hypocyanescent on ICGA) suggests the presence of an active CNV with a small SRF quantity. OCT-A clears up any doubt highlighting the sub-RPE neovascular network.
Therefore, it may happen that the initial diagnosis is CSC without CNV, despite the presence of a small CNV at that time. The clinician should suspect CNV in eyes with one or more of the following: “old age” at onset, a mid/hyperreflective signal under a flat irregular PED, a structure suggestive for CNV on OCT-A (Figure 5), and/or a well-demarcated CNV ‘plaque’ (with or without PCV component) on ICGA [43][147].
Because up to two-thirds of CSC patients with CNV can have a polypoidal component, ICG-A is a keytool for identifying and localizing these polypoidal structures [43][147]. De Salvo et al. [44][148] compared SD-OCT and ICGA levels of sensitivity and specificity in detecting idiopathic PCV and separating PCV from occult CNV. SD-OCT showed a 94.6% sensitivity and a 92.9% specificity.
The standard treatment for CSC complicated by active CNV is intravitreal anti-VEGF, possibly supplemented by HD- or HF-PDT. Several reports demonstrated good efficacy in these cases [45][46][47][48][149,150,151,152]. The MINERVA study found that intravitreal ranibizumab is effective in CNV with an unusual origin, including CNV due to CSC [47][151].
With respect to PCV, large randomized controlled trials based on EVEREST II and PLANET studies found that a combination of full-dose PDT and intravitreal ranibizumab or aflibercept may be beneficial [49][50][153,154]. In addition, it has been recently reported that 50% of PCV lesions were resolved after full-fluence PDT monotherapy, compared to 25% of lesions in patients who received anti-VEGF only [48][152]. As to the group of CSC patients with flat irregular PED in which thin neovascularization can be detected by OCT-A, the use of anti-VEGF therapy should be weighed carefully and deferred until active leakage becomes evident [30][135]. In addition, OCT-A, FA, and/or ICGA should be routinely performed to rule out the possibility of CNV presence in chronic CSC eyes with intraretinal fluid, as up to 45% of these cases may host CNV and should undergo the appropriate therapy [23][128].
OCT and OCT-A implemented significantly information provided by angiographies. SD-OCT is helpful in confirming the diagnosis of acute and chronic CSC and essential in determining the presence of features (SRF volume and height, SRF persistence, photoreceptors integrity, PED number, and subretinal fibrin) able to influence the natural course of the disease or the outcome of treatment procedures. Actually, some of these features are associated with a poor prognosis for final visual acuity. Moreover, nowadays, OCT-A is warmly recommended to identify CNV complicating chronic CSC, so avoiding the risk to miss the diagnosis and administer the wrong treatment.
