Recent advancements in the arena of onco-targeted therapies have enabled safe and effective tumor-specific localization through stimuli-responsive drug delivery systems. Owing to their promising characteristic features, stimuli-responsive drug delivery platforms have revolutionized the chemotherapy-based treatments with added benefits of enhanced bioavailability and selective cytotoxicity of cancer cells compared to the conventional modalities. The insensitivity of stimuli-responsive drug delivery platforms when exposed to normal cells prevents the release of cytotoxic drugs into the normal cells and therefore alleviates the off-target events associated with chemotherapy. Contrastingly, they showed amplified sensitivity and triggered release of chemotherapeutic payload when internalized into the tumor microenvironment causing maximum cytotoxic responses and the induction of cancer cell necrosis.
- tumor
- chemotherapy
- stimuli-responsive drug delivery systems
- prodrugs
1. Introduction
Cancer remains one of the leading causes of death globally. Each year more than eight million people die of cancer worldwide [1]. Despite the significant advancements in the fundamental understanding of cancer biology in the past several decades, the overall mortality of cancer is still high. A major reason for this is our incapability to deliver active pharmaceutical agents selectively to the disease sites without harming healthy tissue. Current treatment strategies for most cancers involve a combination of chemotherapy, radiation therapy and surgical resection. These treatment strategies are non-selective, which lead to significant morbidity and mortality. The primary concern with utilizing the chemotherapeutic agents is their inability to differentiate between healthy and cancer tissue. This is particularly harmful to rapidly growing cells in the body such as hair and soft tissues. The most cytotoxic agents are certainly the most effective but often result in severer adverse effects [2][3][4][2,3,4].
To get optimum effects, suitable drug dosage delivery and the duration of therapy in conjunction with a highly specified drug delivery system is required to be able to release the chemotherapeutic agents in a controlled manner. The major dispute of such a drug delivery system is to deliver the safe and optimum quantities of the drug compared to currently used treatment approaches [5]. Tumor targeting has emerged as an attractive strategy to allow access to tumors and avoid penetration into normal tissue interstitium. Tumor targeting is classified into passive and active targeting; however, the active targeting process occurs only after passive accumulation in tumors [6]. Passive targeting involves enhanced permeability and retention effect (EPR) due to rapid formation of hyper-permeable complex tumor vasculature characterized by impaired lymphatic drainage of diseased tissue (tumor), resulting in the extravasation of ≥100 nm nanoparticles into the tumor microenvironment and preventing their clearance. In contrast, the active targeting strategy is based on the composition decoration of the surface of drug carriers with tumor-specific ligands such as aptamers, antibodies and receptors overexpressed by the tumor cell [7].
Currently, great attention is being given from researchers to nanoscale-based drug delivery systems for the treatment of cancerous cells, such as tunable prodrugs [8], polymeric micelles [9], inorganic nanoparticles [10], nanotubes [11], nanorods [12], dendrimers [13], lipid-based drug delivery systems [14] and carrier-based drug delivery systems [15]. Among the aforementioned drug delivery strategies, stimuli-responsive lipid-based drug delivery systems, nanocarriers and prodrugs as displayed in Figure 1, have attained the greatest attention. Additionally, these drug delivery systems have the imperative attributes which include enhanced selectivity, biocompatibility, cancer microenvironment-based sensitivity and clinical acceptance with added benefits of easy scale up and multiple options regarding the choice and selection of formulation components. Drug carriers/lipids/prodrugs mostly exploit both passive and active strategies of tumor targeting to increase drug bioavailability. Drug delivery systems through stimuli-responsive carriers, lipids and/or prodrugs in the tumor milieu can lead to accelerated/triggered drug release at the target site, improved cellular binding and internalization, or more effective drug perfusion throughout the tumor volume [16]. The modern era has seen the development of multiple stimuli-responsive drug delivery systems that can achieve a several-fold enhancement of tumor necrosis. In addition, biocompatible excipients-based drug delivery systems are utilized for tumor-specific localization, stealth effect and easy clearance from the body. Moreover, advanced drug delivery systems could efficiently facilitate chemotherapy, gene therapy or both for theranostic applications of anti-neoplastics through passive targeting, active targeting, or combined active and passive targeting [17] (Figure 2).
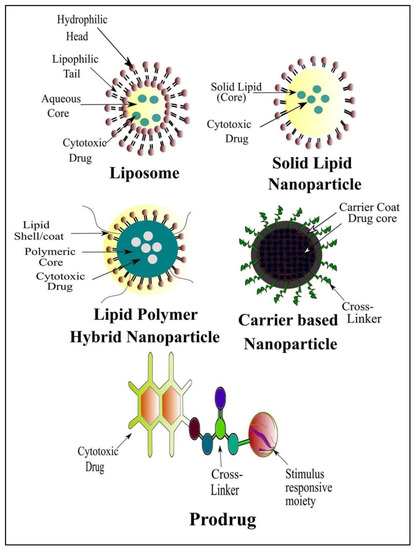
Figure 1.
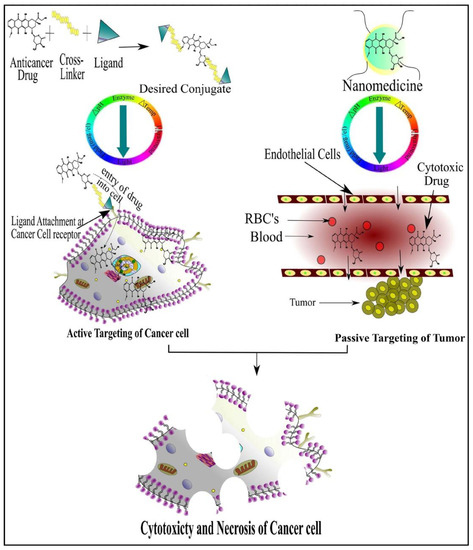
Figure 2.
Cancer Cell-Specific Cytotoxicity by Active and Passive Targeting of Chemotherapeutic Agent(s).
2. Stimuli-Responsive Drug Delivery Systems
Stimuli-based drug delivery systems have constituted a new platform in the understanding of diseases at the molecular level. For this purpose, nanotechnology-based drug delivery systems offer tremendous and promising advantages in the prevention, diagnosis and therapy of many diseases. The stimuli-based drug delivery system includes the phenomenon that influences an activity at a particular site or target tissue to bring about useful activities for the drug release via various mechanisms and is known as “stimuli responsive materials” or “environmentally-responsive materials” [18][19][18,19]. Stimuli-responsive materials are those that undergo a physical or chemical change in response to an external stimulus. These materials exhibit the environment responsive behavior phenomenon and respond to the external stimuli due to biomimetic nature. The stimulus-based drug delivery system is of great importance in the field of nanomedicines and nanotechnology due to controlled and targeted release at the site of action [20]. Stimuli-responsive systems respond to specific triggers to release their cargo at the desired site, hence they can enhance drug efficacy and overcome the adverse effects related to oral or parenteral drug delivery. Drug delivery systems with the ability to respond to temperature, pH change, enzymes, light, magnetic field, ultrasound or electrical stimuli have been heavily investigated over the past few decades [21]. Many intelligent designs of these delivery systems are based on polymers [22], hydrogels [23] and nanoparticles [24]. The stimuli-responsive drug delivery systems may be classified as physical and chemical stimuli-responsive drug delivery systems which are summarized in Table 1.
Table 1. Stimuli-Responsive Drug Delivery Systems for Cancer Chemotherapy.
|
S. No |
Stimulus |
Drug |
Drug Delivery System |
Reference |
|---|---|---|---|---|
|
1. |
Temperature |
Doxorubicin |
Liposomes, Micellar nanoparticles, Hydrogels, Polymeric nanoparticles, Dendrimers |
|
|
Docetaxel |
Micelles, Hydrogel, Liquid Suppository, Liposomes |
|||
|
2. |
Magnetic Field |
Doxorubicin |
Magneto-liposomes, FeCo/Graphite shell Nanocrystals, Alginate embedded Magnetic Nanoheaters, Magnetic iron oxide nanoparticles |
|
|
Docetaxel |
Docetaxel grafted magnetic nanoparticles, Nanocomposite, Polymeric iron oxide nanoparticles |
|||
|
3. |
Electric Field |
Antisense oligonucleotides |
Liposome nanoparticles, Hydrogels |
|
|
4. |
Ultrasound |
Doxorubicin |
Polypeptide doxorubicin nanoconjugates, Alginate nanodroplets, PEGylated Liposomes, Microbubbles |
|
|
Docetaxel |
Nanobubbles, Lipid microbubbles, |
|||
|
5. |
Light |
Doxorubicin |
Gold nanospheres, Stealth Liposomes, Micelles, Mesoporous silica nanocarriers, Nanogels, |
|
|
Docetaxel |
PEGylated Gold Nanorod Coated Poly(l-lactide) Microneedles, Nanocomposites, |
|||
|
6. |
pH |
Doxorubicin |
Nanogels, Liposomes, Magnetic chitosan nanoparticles, Microgels, Micelles, Mesoporous silica nanoparticles, Magnetic nanoparticles, Dendrimers |
|
|
Docetaxel |
Liposomes, Lipid polymer hybrid nanoparticles, Mesoporous carbon nanoparticles, Micelles |
|||
|
7. |
Enzymes |
Doxorubicin |
Magnetic iron-oxide nanoparticles, Polymer-peptide-drug conjugates, Nanofibers, Dendrimers |
|
|
Paclitaxel |
Polymeric nanoparticles, Solid lipid nanoparticles, Dendrimers, Micelles |
3. Molecular Dynamics (MD) Simulations Associated with Stimuli-Based Tumor Targeting
The rational release of drugs from multi-functional nanoparticles can be precisely predicted by the use of multiscale computational modeling. These mechanistic models can better provide the knowledge regarding the fundamental interactions between drug, carriers and patient. However, this multiscale computational modeling is in its early days for traditional drug delivery. For emerging nanotechnology systems for drug delivery, a reliable in silico prediction is needed. In the past, the drug development stages have shown that ~39% of failures are due to poor pharmacokinetics and ~11% are due to toxicity. So, the in silico prediction of the pharmacokinetics of potential new entities in nascent stages of development has become a critical area of research nowadays. Molecular interaction between nano/micro-scale and drug, vehicles, GIT and target site requires computational chemistry approaches such as molecular dynamics (MD) and mesoscale modeling. Stochastic approaches are employed to predict the effect of variance that arises from biological sources both at macro and micro/nano levels [74][75][291,292].
In stimuli-responsive systems, the use of molecular dynamics simulations is an emerging field with great potential. The experimental findings can be better simulated by the use of atomistic modeling, and particularly all-atom molecular dynamics (MD) simulations [76][293]. This high molecular simulation will provide the unique detail about the polymer structure, its interaction with solvents and structural changes that occurred with stimuli. Using MD simulations, the effect of pH not only provides insight into any structural changes in dendrimers but also the possible interaction of the dendrimers with other molecular targets such as siRNA (Figure 3A) [77][78][294,295]. Similarly, carter et al., 2014 [33], identified a novel light-absorbing monomer by using molecular dynamics simulations which give rise to a more stable porphyrin bilayer across liposomes. Thus, light-induced membrane permeation was enabled with inclusion of 10 molar % porphyrin-phospholipid within liposome formulations. Nguyen et al., 2011 [79][296], performed MD simulations in a full-atom model to provide a clear perception about possible interaction between chitosan and exendin-4 in the presence/absence of Fe3+. In that study, nine exendin-4 and four 20-mer chitosan molecules were used in the MD simulation at pH 6.5, in which the ratio of deprotonated (–NH2) and protonated (–NH3+) amine groups on chitosan was about 1:1. The pI (phosphoinositide) of exendin-4, containing five glutamic acids (Glu) and one aspartic acid (Asp), is around 4.2. The result showed that in the absence of Fe3+, an electrostatic interaction was formed between two sets of complexes, each containing two chitosan and three exendin-4 molecules, while three exendin-4 molecules were left alone. In contrast, in the presence of iron, a single complex consisting of four chitosan and nine exendin-4 molecules was found due to coordinate-covalent bonds in the locality of nitrogen as well as carboxylic, glycosidic, and hydroxyl oxygen atoms. Therefore, apart from electrostatic interactions, in the presence of Fe3+, coordinate-covalent bonds were formed between chitosan and exendin-4, which significantly increased the drug loading in the preparation of test nanoparticles (Figure 3B). Sekar et al., 2018 [80][297], performed the in silico molecular docking studies to examine the possible interaction of chitosan ascorbate nanoparticles (CANs) with significant targets of cervical cancer. Eighty sets of docked complexes were generated by docking of each enzyme with chitosan ascorbate nanoparticles (Figure 3C). The highest and lowest binding energy for the docking of CANs with 10 enzymes were determined. These different proteins were associated with different cell signaling pathways, causing rapid cell growth, neo-vascularization, abnormal apoptosis and cell migration in cervical cancer. Based on the docking analysis, the binding ability of chitosan ascorbate nanoparticles with potent cervical cancer protein targets was confirmed. The interactions of molecules concluded that CANs mimic the binding of substrate to these enzymes. Wang et al., 2010 [40], applied Monte Carlo Simulations to design nanoparticles for enhanced tumor targeting. The modeling was applied on three different parameters and it was concluded that, by increasing numbers of ligands, binding energy and length of polymer chain of nanoparticles, specific tumor targeting for longer periods could be achieved. Yang et al., 2010 [81][298], investigated the physical translocation of nanoparticles with different shapes and volumes across a lipid bilayer by applying computer simulations. In spherical shape, the hydrophobicity and large size of nanoparticles resulted in enhanced permeation and targeting through optimum binding energy. By contrast, in pin like shape, the energy was not required but the size was small. Duncan et al., 2015 [82][299], applied computer simulation to nanoparticle drug delivery for selective targeting. This study was based on the affinity and selectivity of nanoparticles towards cancerous cells and represented with β. When β is equal to −1, it indicates high selectivity and weak binding to cancer cells. When β = 1, it indicates low selectivity and strong binding, while, when β = 0, it indicates a balance between binding and selectivity which is optimal for cancer cell targeting (Figure 3D). The results demonstrated that nanoparticles offered more selectivity towards cancer cells compared to the normal cell. In addition, nanoparticles showed enhanced binding with cancer cells and slight or negligible binding to normal cells.
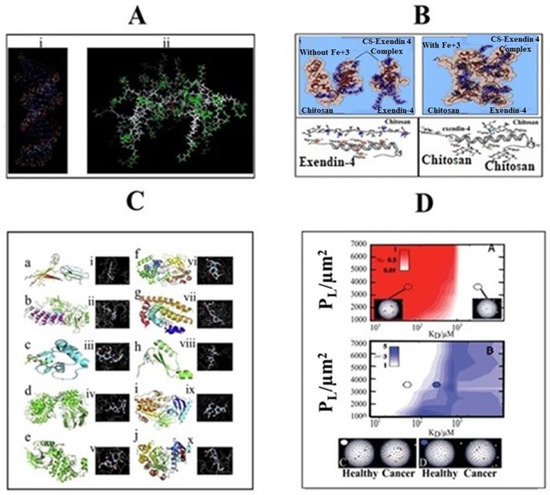
Figure 3. Molecular Dynamic Simulation of (A) siRNA-based dendrimers, (B) interaction between 4 20-mer chitosan (CS) and 9 exendin-4 molecules in the absence/presence of Fe3+ at pH 6.5, (C) docking scheme 2D model representing the affinity and (D) selectivity of nanoparticles towards healthy and cancerous cells (adopted with Copyright permission).
