4Inelastic neutron scattering (INS) is a spectroscopy based on the energy analysis of neutrons after they have been scattered by a sample. A detected energy transfer can be related to a physical interaction of the corresponding atoms with their environment. An energy transfer of several meVs typically arises from vibrations of atoms. Thus, INS provides an amplitude-of-motion and neutron incoherent cross section weighted phonon density of states. Given the much higher incoherent scattering cross section of hydrogen relative to that of all other elements, INS is particular sensitive to hydrogen based vibrations. The method is widely used in condensed matter physics and solid state chemistry, because the vibrational properties of matter define various physical properties such as the heat capacity. If used as a fingerprint method, INS can be used to characterize chemical bonds both in the bulk as well as on the surface.
- inelastic neutron scattering
- vibrational properties
- phonons
- adsorbates
- hydrogen
- infrared spectroscopy
- Raman spectroscopy
Inelastic neutron scattering (INS) is a powerful technique to study the vibrational properties of materials and gives insights into the bonding and other properties[1]. The method exploits the slightly different behaviour between bound and free atoms during the scattering process (see Fig. 1) and uses the difference in energy and momentum transfer to construct the vibrational states of the system [1].
Fig. 1: Sketch of the inelastic scattering of a neutron by a hydrogen molecule. The energy loss corresponds to the energy taken up by the molecule.
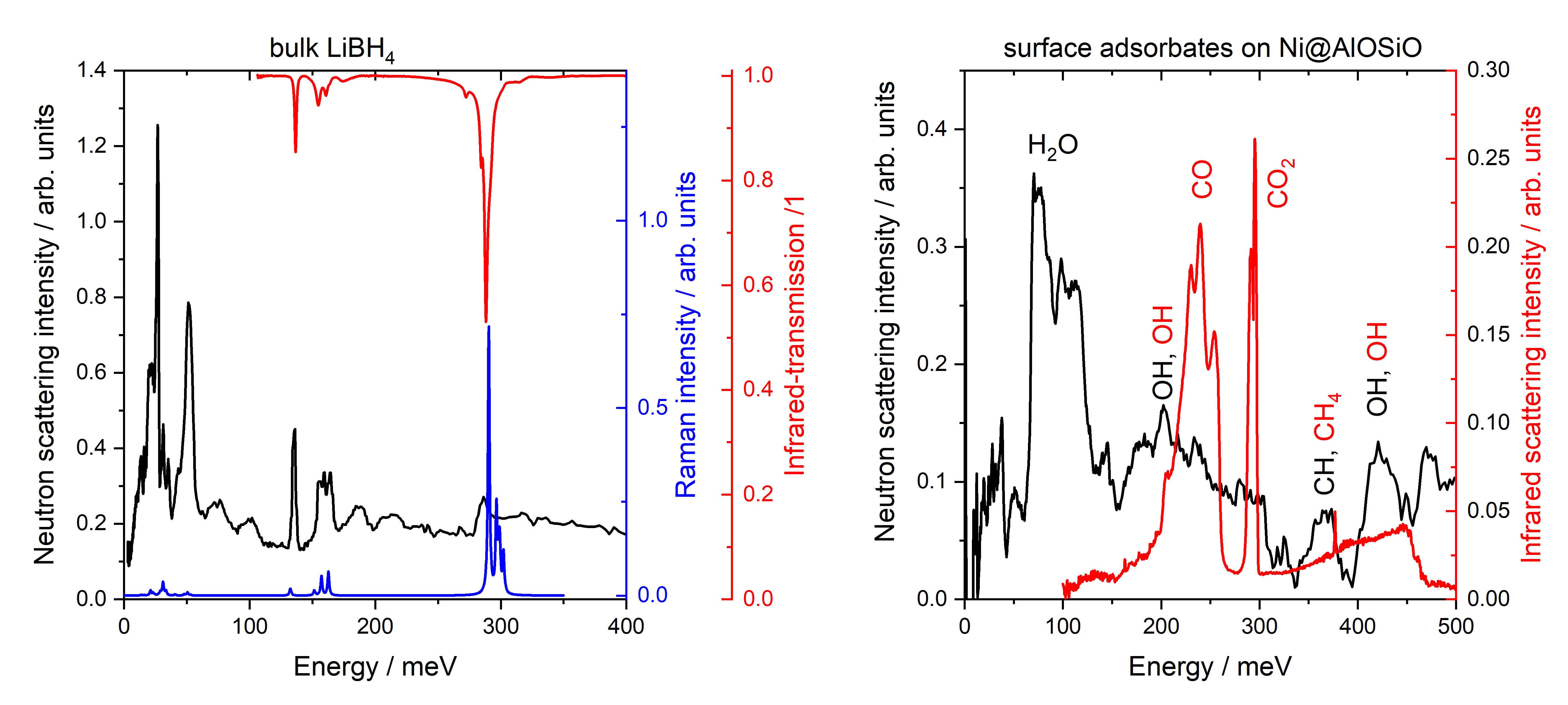
The advantage of INS compared to the related Raman scattering (i.e., inelastic photon scattering) and infrared spectroscopies (IR absorption and reflectivity) is its independence on optical selection rules[2]. Moreover, phonons (lattice vibrations) in metals cannot readily be detected by IR techniques[3], and Raman scattering from metals is weak due to the screening of electromagnetic radiation by quasi-free electrons [2]. In contrast, neutrons do not interact with electrons and sample the entire Brillouin zone (different k-vector and momentum transfer), while Raman and IR are restricted to the zone center (Γ-point). Thus, the full vibrational spectra of many metal hydrides have been exclusively derived from INS measurements (e.g., Refs.[1],[4],[5]). Fig. 2 shows along the example LiBH4, how all three spectroscopies can be used to probe the vibrational properties. INS is particularly sensitive at low energy transfers, which is difficult for infrared spectroscopy. The comparison elucidates the dependence on optical selection rules: e.g., the peak around 50 meV originating from librational modes is hardly detected by Raman spectroscopy, but one of the strongest INS peaks.
From density-functional theory (DFT), the electronic structure of matter can be calculated, from which the physical and chemical properties are derived and compared to experimental measurements. Equally, the vibrational properties can be calculated by DFT. The consistency of calculated and experimental vibrational properties may used as a benchmark for the understanding of the system under investigation.
Also surface vibrational states are accessible by INS [1],[6]. However, the INS-signal is rather weak, in particular if compared to optical methods such as DRIFTS being sensitive to photons scattered by the surface [6] . On the other hand, the presence of a variety of surface adsorbates as typical in catalysis impedes the interpretation of DRIFTS spectra being non-selective to the chemistry of the compounds. Here, the application of the hydrogen-selective, but post-mortem method INS supports the analysis of results from operando DRIFTS[6] (Fig. 2).
Fig. 2: Examples of the INS spectra applied on the bulk system LiBH4 (left, from Ref. [7]) and on surface states present during CO2 methanation on a Ni-catalyst (right, from Ref. [6]). To highlight the similarities and differences of INS to other vibrational spectroscopies, IR-transmission, DRIFTS, and Raman spectra are also shown where applicable.
For LiBH4, both infrared as well as INS spectra are dominated by hydrogen based vibrations. This is different for the catalyst: here, the DRIFTS spectrum is dominated by CO2 and CO vibrations, while INS shows the vibrations of hydrogen based compounds.
As the method requires the generation and analysis of neutrons, which is possible in large sacel facilities only, the method is usually used as an acid test after the application of other, less demanding spectroscopic techniques such as DRIFTS.
References
- Mitchell, P. C. H.; Parker, S. F.; Ramirez-Cuesta, A. J.; Tomkinson, J. . Vibrational Spectroscopy with Neutrons With Applications in Chemistry, Biology, Materials Science and Catalysis; World Scientific Publishing: Singapore, 2005; pp. 1.
- Brüesch, P.. Phonons: Theory and Experiments II: Experiments and Interpretation of Experimental Results; Springer: Heidelberg, 1986; pp. 1.
- Rode, M.; Borgschulte, A.; Jacob, A.; Stellmach, C.; Barkow, U.; Schoenes J.; Evidence for Ionic Bonding in YH3. Physical Review Letters 2001, 87, 235502, 10.1103/physrevlett.87.235502.
- Alexander I. Kolesnikov; V.E. Antonov; V.K. Fedotov; G. Grosse; A.S. Ivanov; F.E. Wagner; Lattice dynamics of high-pressure hydrides of the group VI–VIII transition metals. Physica B: Condensed Matter 2002, 316, 158-161, 10.1016/s0921-4526(02)00447-7.
- Jasmin Terreni; Olga Sambalova; Andreas Borgschulte; Svemir Rudić; Stewart F. Parker; Anibal Ramirez-Cuesta; Volatile Hydrogen Intermediates of CO2 Methanation by Inelastic Neutron Scattering. Catalysts 2020, 10, 433, 10.3390/catal10040433.
- Andreas Borgschulte; Jasmin Terreni; Emanuel Billeter; Luke Daemen; Yongqiang Cheng; Anup Pandey; Zbigniew Łodziana; Russell J. Hemley; Anibal Ramirez-Cuesta; Inelastic neutron scattering evidence for anomalous H–H distances in metal hydrides. Proceedings of the National Academy of Sciences 2020, 117, 4021-4026, 10.1073/pnas.1912900117.
- A. Borgschulte; A Jain; A J Ramirez-Cuesta; P Martelli; A Remhof; O. Friedrichs; R. Gremaud; A Züttel; Mobility and dynamics in the complex hydrides LiAlH4 and LiBH4.. Faraday Discussions 2011, 151, 213.