Neurodegenerative diseases resulting from the progressive loss of structure and/or function of neurons contribute to different paralysis degrees and loss of cognition and sensation. The lack of successful curative therapies for neurodegenerative disorders leads to a considerable burden on society and a high economic impact. Over the past 20 years, regenerative cell therapy, also known as stem cell therapy, has provided an excellent opportunity to investigate potentially powerful innovative strategies for treating neurodegenerative diseases.
- stem cells
- therapy
- regenerative
- neurodegenerative diseases
- Parkinson’s disease
- Huntington’s disease
1. Introduction
Neurodegenerative disorders, such as Parkinson’s disease (PD), Alzheimer’s disease (AD), Huntington’s disease (HD), amyotrophic lateral sclerosis (ALS), and frontotemporal dementia (FTD), are characterized by a progressive loss of structure, function, or number of neurons in the brain or spinal cord. Unfortunately, the currently available treatment options are insufficient in arresting the neurodegenerative processes [1]. The complexity of the mechanisms associated with neuronal loss and the contradicting physiological causes of these diseases significantly hinder our understanding of the pathogenic processes and the consequential development of effective treatments [2]. Moreover, difficulty in targeting the widespread neuronal cell death, coupled with the lack of robust regenerative capacity of the central nervous system (CNS) and the enormous limitations for the vast majority of drugs (98% of small-molecule drugs and 100% of large-molecule drugs) regarding crossing the blood–brain barrier (BBB) further adds to the difficulty of treating these diseases [3][4][5][6][7][3–7]. The loss of quality of life, the cost of care, and the lack of effective therapies are an enormous burden for over 7 million people in the USA living with these neurodegenerative diseases [8].
Stem cell therapy, also known as regenerative therapy, improves the repair response of dysfunctional and damaged tissue using stem cells or their derivatives. The objectives of stem cell therapies typically focus either on cellular replacement or on providing environmental enrichment. Stem cell therapy has revolutionized medicine over the years since its therapeutic applications have provided invaluable and attractive options for treating numerous disorders, including neurodegenerative diseases [9]. The potential of stem cell therapy in neurodegenerative diseases was first examined in the 1980s when patients suffering from PD were treated with fetal mesencephalic tissue transplantation [10]. Nowadays, stem cell therapy offers promising strategies for treating almost all forms of neurodegenerative disorders. These strategies involve the regeneration of neural tissue, stabilizing the neuronal networks, providing neurotrophic support, and alleviating neurodegeneration at different neuronal circuitry levels [9]. Scientists are continually trying to find sturdy, safe, and readily available stem cell sources while refining and/or developing new delivery methods to improve the treatment's efficiency and effectiveness and reduce the side effects [11].
2. Stem Cell Classifications
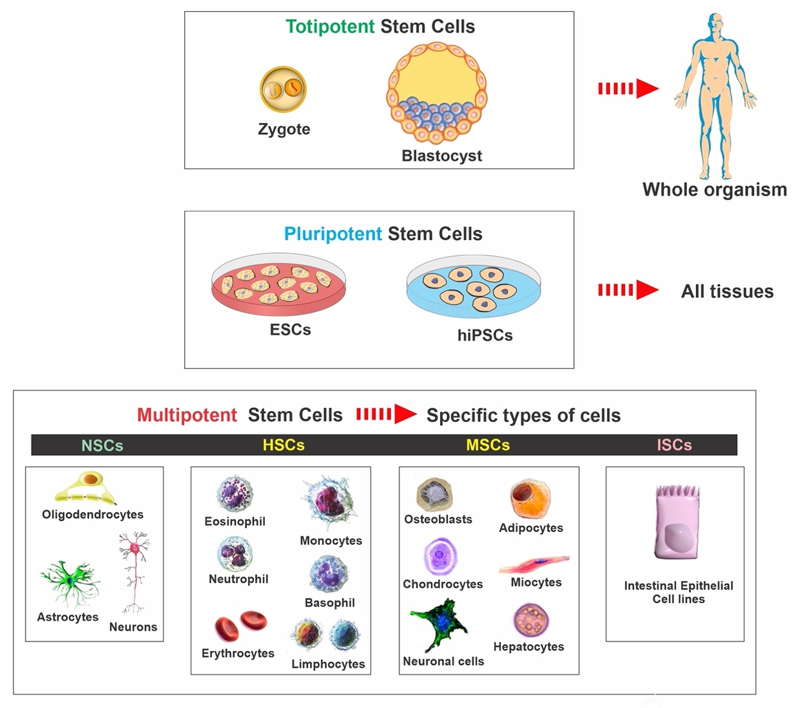
Stem cells are characterized by the capacity to proliferate, self-renew, and differentiate into various mature cell lineages. There are different classifications of stem cells, including embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), mesenchymal stem cells (MSCs), and neural stem cells (NSCs). The classification is based on the range of possible cell type production and derivation methods. Therefore, it is essential to understand the characteristics of the various available stem cell types and the potential effect of cellular therapies on disease mechanisms (see Figure 1). The rationale for utilizing each type of stem cell depends on the desired applications and outcomes since each type possesses individual qualities and advantages. In the following paragraphs, we summarized the various types and general aspects of stem cells used in basic research and clinical trials (see Table 1).
Figure 1. Classification of stem cells: ESCs—embryonic stem cells, hiPSCs—human induced pluripotent stem cells, NSCs—neural stem cells, HSCs—hematopoietic stem cells, MSCs—mesenchymal stem cells, ISCs—intestinal stem cells.
Table 1. Comparison between the various types of stem cells. This side-by-side comparison includes their origin and the inherent clinical advantages and disadvantages of using these cells.
|
Stem Cell Type |
Origin |
Advantages |
Disadvantages |
|
ESCs (pluripotent) |
Embryo (blastocyst) |
ü Unlimited proliferation |
ü Ethical problems ü Risk of immune rejection ü Unpredictable differentiation ü High risk of tumor formation |
|
IPSCs (pluripotent) |
Reprogrammed adult cells: fibroblasts, hepatocytes, circulating T cells, and keratinocytes |
ü No ethical problems ü Low risk of immune rejection ü High accessibility |
ü High risk of tumor formation ü Risk of susceptibility to the original pathology of the patient ü Genetic and epigenetic abnormalities |
|
MSCs (multipotent) |
Adult tissues (bone marrow, skin, blood, umbilical cord, etc.) |
ü No ethical problems ü High accessibility ü Easy isolation methods ü Autologous cells generation ü Self-renewal capacity ü Low risk of immune rejection |
ü Risk of tumor formation |
|
NSCs (Multipotent) |
Embryo, human fetal brain and brain tissue of adults (SVZ and SGZ of hippocampus) |
ü Low risk of tumor formation
|
ü Ethical problems ü Risk of immune rejection ü Limited differentiation ü Low self-renewal capacity ü Limited proliferation and expansion ü Limited availability ü Difficult isolating methods |
SGZ: subgranular zone, SVZ: subventricular zone.
2.1. Embryonic Stem Cells
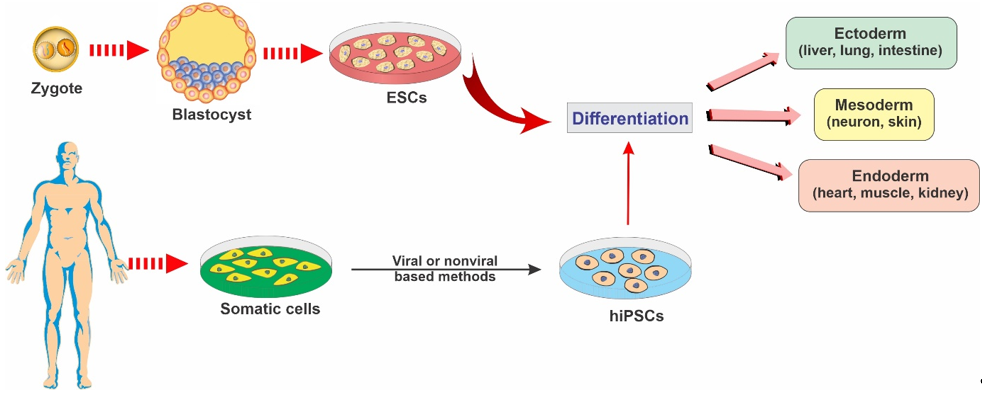
ESCs are a class of pluripotent stem cells derived from the inner cell mass of blastocysts (an embryo that has been left to develop for 5 to 6 days and presents a relatively complex cellular structure formed of approximately 100–200 cells; see Figure 2). ESCs offer promising avenues for research due to their ability to self-renew indefinitely and differentiate into almost all cell types of the central nervous system. These cells are currently being used as an invaluable cell source of human neuronal progenitors in large quantities and high purity in various research areas related to neurodegenerative diseases [12].[12]
Researchers are currently focusing heavily on the therapeutic potential of ESCs. Although ESCs offer new means of treatment, it still raises some thorny ethical and religious restrictions since it involves destroying human embryos [4][13][4,13]. Additionally, there are several medical concerns associated with all novel ESC therapies in translational medicine, such as the significant risk of immunorejection in the host patient, as well as tumor formation and cancer as a result of the persistence of nondifferentiated cells undergoing malignant transformation and genetic instability following a prolonged time in culture [14].
Figure 2. Representative diagram depicting the main types of stem cells and their potential to differentiate into various lineages.
2.2. Induced Pluripotent Stem Cells
iPSCs are a type of pluripotent stem cells that are artificially derived from non-pluripotent, adult somatic cells, including fibroblasts, hepatocytes, circulating T cells, and keratinocytes, by forcing the expression of genes and transcription factors that maintain embryonic stem cells [15]. These reprogrammed cells now provide a promising strategy for producing unlimited autologous neurons for transplantation in neurodegenerative patients [2]. iPSCs can be converted into mature functional neural lineages using an optimized differentiation method, which widens the scope of its potential applications in the studies of the mechanisms underlying various neurodegenerative disorders and the screening of novel therapeutic targets [16][17][16,17]. For example, in a patient with a neurodegenerative disease, a pluripotent cell can be taken from the skin or blood (see Figure 2). The resulting iPSCs can become a reliable source for generating those neural cells affected by degenerative brain disease [18].
One of the main distinct advantages of iPSCs is the lack of ethical and religious implications because cells can be produced without oocytes or embryos. Another significant merit of iPSCs is that they can be generated from the patients themselves, thus providing a valuable avenue for autologous cell transplantation with no risk of immune rejection and no need for immunosuppressive agents [19][20][19,20]. iPSCs offer potential clinical advantages due to more straightforward harvesting methods and fewer possible side effects, with better specific terminally differentiated cell phenotypes. However, the differentiation of IPSCs into mature neurons is more complicated than for ESCs. Like ESCs, there is still the risk of tumor formation due to unwanted viral integration, causing chromosomal disruptions and mutations and low reprogramming efficiency during these cells' production [14][21][14,21]. Hence, the clinical application of IPSCs in neurodegenerative diseases is still not feasible yet, owing to the lack of in-depth research evaluating its therapeutic safety among human subjects.
2.3. Mesenchymal Stem Cells
MSCs, which are traditionally found in the bone marrow, umbilical cord, adipose tissue, and spleen, are adult, self-renewing, multipotent stem cells that can differentiate into various cell types, including bone, cartilage, fat, and muscle [22]. MSCs have enormous therapeutic potential and could be an ideal source for cell transplantation in neurodegenerative diseases due to their excellent self-renewal capacity while maintaining multipotency [23]. MSC-derived functional neurons appear to be more promising regarding neurodegenerative diseases than ESCs due to the relatively easy collecting methods and fewer related ethical, religious, and immunorejection concerns [24]. Furthermore, MSCs do not organize tumors like other primitive stem cells, such as ESCs [25]. Thus, the promising abilities of MSCs present them as an attractive platform for research into neurodegenerative disorders. Several studies have also indicated that MSCs might possess the ability to cross the BBB, which is crucial for the proper delivery of neurotherapeutic agents into the CNS [26][27][26,27]. It has been shown that MSCs can cross the BBB through paracellular pathways, despite the presence of tight junctions that would normally block such passages [28]. There are preclinical studies and ongoing clinical trials currently assessing the therapeutic effectiveness of MSCs in various neurodegenerative diseases. MSCs are delivered via either intracerebral or intrathecal injections. Following transplantation, MSCs initiate their neuroregenerative function, including promoting neuronal growth, producing neurotrophic factors, stimulating endogenous neurogenesis, activating microglia, suppressing inflammation, and decreasing apoptosis and free radicals [18]. MSCs can also secrete angiopoietin-1, angiogenic cytokines, and extracellular matrix components, thus improving angiogenesis and promoting the recruitment of neural progenitor cells (NPCs) [29].
2.4. Neural Stem Cells
NSCs are multipotent stem cells in brain tissue that are more specialized than ESCs. NSCs have a decreased potential for self-renewal and usually differentiate into only limited cell lineage of the brain tissue, including oligodendrocytes, neurons, and astrocytes [13][30][13,30] (see Figure 2). NSCs can be derived from various regions of both the embryonic and the human fetal brain or the brain tissue of patients undergoing surgical therapies [30][31][32][33][30–33].
The transplantation of NSCs to other brain regions is considered a possible therapeutic avenue for the treatment of many neurodegenerative diseases [1][34][1,34]. For example, NSCs can play a role in gliogenesis by releasing bioactive molecules that regulate neuronal excitability, synaptic activity, and plasticity [35]. NSCs can also generate and release synergistic and antagonistic molecules, triggering intracellular NSC regulatory mechanisms, such as transcription factors, epigenetic responses, and metabolism [36]. Furthermore, NSCs can establish synaptic connections with surrounding neurons, integrate into existing circuitry, and repair the impaired network [37]. Of note, unlike ESCs, NSCs are considered genetically stable and less tumorigenic. The low self-renewal potential of NSCs can be resolved via the genetic modification of these cells to produce immortalized NSCs with enhanced proliferative potential [38]. However, there are still significant obstacles for the therapeutic application of NSCs due to the inevitable possibility of immunological incompatibility in allogeneic transplantation, limited sources, difficulties in isolating these cells, limited proliferation and expansion, and ethical and religious issues [39].
References
- De Gioia, R.; Biella, F.; Citterio, G.; Rizzo, F.; Abati, E.; Nizzardo, M.; Bresolin, N.; Comi, G.P.; Corti, S. Neural Stem Cell Transplantation for Neurodegenerative Diseases. J. Mol. Sci. 2020, 21, 3103.
- Chang, C.-Y.; Ting, H.-C.; Liu, C.-A.; Su, H.-L.; Chiou, T.-W.; Lin, S.-Z.; Harn, H.-J.; Ho, T.-J. Induced pluripotent stem cell (iPSC)-based neurodegenerative disease models for phenotype recapitulation and drug screening. Molecules 2020, 25, 2000.
- Helms, H.C.; Abbott, N.J.; Burek, M.; Cecchelli, R.; Couraud, P.-O.; Deli, MA; Förster, C.; Galla, H.J.; Romero, I.A.; Shusta, EV In vitro models of the blood–brain barrier: An overview of commonly used brain endothelial cell culture models and guidelines for their use. Cereb. Blood Flow Metab. 2016, 36, 862–890.
- Sivandzade, F.; Cucullo, L. In-vitro blood–brain barrier modeling: A review of modern and fast-advancing technologies. Cereb. Blood Flow Metab. 2018, 38, 1667–1681.
- Tam, V.H.; Sosa, C.; Liu, R.; Yao, N.; Priestley, R.D. Nanomedicine as a noninvasive strategy for drug delivery across the blood brain barrier. J. Pharm. 2016, 515, 331–342.
- Wen, M.M.; El-Salamouni, N.S.; El-Refaie, W.M.; Hazzah, H.A.; Ali, M.M.; Tosi, G.; Farid, R.M.; Blanco-Prieto, M.J.; Billa, N.; Hanafy, A.S. Nanotechnology-based drug delivery systems for Alzheimer’s disease management: Technical, industrial, and clinical challenges. Control. Release 2017, 245, 95–107.
- Zhang, B.; Yan, W.; Zhu, Y.; Yang, W.; Le, W.; Chen, B.; Zhu, R.; Cheng, L. Nanomaterials in neural‐stem‐cell‐mediated regenerative medicine: Imaging and treatment of neurological diseases. Mater. 2018, 30, 1705694.
- Lunn, J.S.; Sakowski, S.A.; Hur, J.; Feldman, E.L. Stem cell technology for neurodegenerative diseases. of Neurol. 2011, 70, 353–361.
- Sakthiswary, R.; Raymond, A.A. Stem cell therapy in neurodegenerative diseases: From principles to practice. Neural Regen. Res. 2012, 7, 1822.
- Shariati, A.; Nemati, R.; Sadeghipour, Y.; Yaghoubi, Y.; Baghbani, R.; Javidi, K.; Zamani, M.; Hassanzadeh, A. Mesenchymal stromal cells (MSCs) for neurodegenerative disease; a promising frontier. J. Cell Biol. 2020, 151097.
- Frenette, P.S.; Pinho, S.; Lucas, D.; Scheiermann, C. Mesenchymal stem cell: Keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Rev. Immunol. 2013, 31, 285–316.
- Drouin-Ouellet, J.; Barker, R.A. Stem cell therapies for Parkinson’s disease: Are trials just around the corner? Med. 2014, 9, 553–555.
- Dekmak, A.; Mantash, S.; Shaito, A.; Toutonji, A.; Ramadan, N.; Ghazale, H.; Kassem, N.; Darwish, H.; Zibara, K. Stem cells and combination therapy for the treatment of traumatic brain injury. Brain Res. 2018, 340, 49–62.
- Kazmerova, Z.; Zilka, N.; Cente, M.; Neradil, P.; Zilkova, M.; Novak, M. Can we teach old dogs new tricks? Neuroprotective cell therapy in Alzheimer’s and Parkinson’s disease. Alzheimer’s Dis. 2013, 37, 251–272.
- de Lázaro, I.; Yilmazer, A.; Kostarelos, K. Induced pluripotent stem (iPS) cells: A new source for cell-based therapeutics? Control. Release 2014, 185, 37–44.
- Sproul, A.A. Being human: The role of pluripotent stem cells in regenerative medicine and humanizing Alzheimer’s disease models. Asp. Med. 2015, 43, 54–65.
- Matsumoto, T.; Fujimori, K.; Andoh-Noda, T.; Ando, T.; Kuzumaki, N.; Toyoshima, M.; Tada, H.; Imaizumi, K.; Ishikawa, M.; Yamaguchi, R. Functional neurons generated from T cell-derived induced pluripotent stem cells for neurological disease modeling. Stem Cell Rep. 2016, 6, 422–435.
- Wang, Y.; Ji, X.; Leak, RK; Chen, F.; Cao, G. Stem cell therapies in age-related neurodegenerative diseases and stroke. Ageing Res. Rev. 2017, 34, 39–50.
- Verma, A.; Verma, N. Induced pluripotent stem cells and promises of neuroregenerative medicine. India 2011, 59, 555.
- Z Barkho, B.; Zhao, X. Adult neural stem cells: Response to stroke injury and potential for therapeutic applications. Stem Cell Res. Ther. 2011, 6, 327–338.
- Sonntag, K.-C.; Song, B.; Lee, N.; Jung, J.H.; Cha, Y.; Leblanc, P.; Neff, C.; Kong, SW; Carter, B.S.; Schweitzer, J. Pluripotent stem cell-based therapy for Parkinson’s disease: Current status and future prospects. Neurobiol. 2018, 168, 1–20.
- Glat, MJ; Offen, D. Cell and gene therapy in Alzheimer’s disease. Stem Cells Dev. 2013, 22, 1490–1496.
- Chen, X.; Wang, S.; Cao, W. Mesenchymal stem cell-mediated immunomodulation in cell therapy of neurodegenerative diseases. Immunol. 2018, 326, 8–14.
- Vissers, C.; Ming, G.-l.; Song, H. Nanoparticle technology and stem cell therapy team up against neurodegenerative disorders. Drug Deliv. Rev. 2019, 148, 239–251.
- Aleynik, A.; Gernavage, K.M.; Mourad, Y.S.; Sherman, L.S.; Liu, K.; Gubenko, Y.A.; Rameshwar, P. Stem cell delivery of therapies for brain disorders. Transl. Med. 2014, 3, 24.
- Li, Z.; Fan, D.; Xiong, D. Mesenchymal stem cells as delivery vectors for anti-tumor therapy. Stem cell investigation 2015, 2.
- Hasan, A.; Deeb, G.; Rahal, R.; Atwi, K.; Mondello, S.; Marei, HE; Gali, A.; Sleiman, E. Mesenchymal stem cells in the treatment of traumatic brain injury. Neurol. 2017, 8, 28.
- Matsushita, T.; Kibayashi, T.; Katayama, T.; Yamashita, Y.; Suzuki, S.; Kawamata, J.; Honmou, O.; Minami, M.; Shimohama, S. Mesenchymal stem cells transmigrate across brain microvascular endothelial cell monolayers through transiently formed inter-endothelial gaps. Lett. 2011, 502, 41–45.
- Kar, M.; Shih, Y.-R.V.; Velez, D.O.; Cabrales, P.; Varghese, S. Poly (ethylene glycol) hydrogels with cell cleavable groups for autonomous cell delivery. Biomaterials 2016, 77, 186–197.
- Murrell, W.; Palmero, E.; Bianco, J.; Stangeland, B.; Joel, M.; Paulson, L.; Thiede, B.; Grieg, Z.; Ramsnes, I.; Skjellegrind, H.K. Expansion of multipotent stem cells from the adult human brain. PLoS ONE 2013, 8, e71334.
- Belenguer, G.; Domingo-Muelas, A.; Ferrón, S.R.; Morante-Redolat, J.M.; Fariñas, I. Isolation, culture and analysis of adult subependymal neural stem cells. Differentiation 2016, 91, 28–41.
- Harris, L.; Zalucki, O.; Piper, M.; Heng, J.I.-T. Insights into the biology and therapeutic applications of neural stem cells. Stem Cells Int. 2016, 2016, 9745315.
- Carradori, D.; Eyer, J.; Saulnier, P.; Préat, V.; Des Rieux, A. The therapeutic contribution of nanomedicine to treat neurodegenerative diseases via neural stem cell differentiation. Biomaterials 2017, 123, 77–91.
- Li, X.; Peng, Z.; Long, L.; Tuo, Y.; Wang, L.; Zhao, X.; Le, W.; Wan, Y. Wnt4‐modified NSC transplantation promotes functional recovery after spinal cord injury. FASEB J. 2020, 34, 82–94.
- Araque, A.; Carmignoto, G.; Haydon, P.G.; Oliet, S.H.; Robitaille, R.; Volterra, A. Gliotransmitters travel in time and space. Neuron 2014, 81, 728–739.
- Bond, A.M.; Ming, G.-l.; Song, H. Adult mammalian neural stem cells and neurogenesis: Five decades later. Cell Stem Cell 2015, 17, 385–395.
- Ming, G.-l.; Song, H. Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron 2011, 70, 687–702.
- Nam, H.; Lee, K.-H.; Nam, D.-H.; Joo, K.M. Adult human neural stem cell therapeutics: Current developmental status and prospect. World J. Stem Cells 2015, 7, 126.
- López-León, M.; Outeiro, T.F.; Goya, R.G. Cell reprogramming: Therapeutic potential and the promise of rejuvenation for the aging brain. Ageing Res. Rev. 2017, 40, 168–181.