Soft-tissue structures are in close interaction with mineralized bone, but also dentine, cementum and enamel of our teeth. These are exposed to intense mechanical and chemical stress as well as to dense microbiologic colonization. Teeth are susceptible to damage, most commonly to caries, where microorganisms from the oral cavity degrade the mineralized tissues of enamel and dentine and invade the soft connective tissue at the core, the dental pulp. However, the pulp is well-equipped to sense and fend off bacteria and their products and mounts various and intricate defense mechanisms. The front rank is formed by a layer of odontoblasts, which line the pulp chamber towards the dentine. These highly specialized cells not only form mineralized tissue but exert important functions as barrier cells. They recognize pathogens early in the process, secrete antibacterial compounds and neutralize bacterial toxins, initiate the immune response and alert other key players of the host defense. As bacteria get closer to the pulp, additional cell types of the pulp, including fibroblasts, stem and immune cells, but also vascular and neuronal networks, contribute with a variety of distinct defense mechanisms, and inflammatory response mechanisms are critical for tissue homeostasis. Still, without therapeutic intervention, a deep carious lesion may lead to tissue necrosis, which allows bacteria to populate the root canal system and invade the periradicular bone via the apical foramen at the root tip. The periodontal tissues and alveolar bone react to the insult with an inflammatory response, most commonly by the formation of an apical granuloma. Healing can occur after pathogen removal, which is achieved by disinfection and obturation of the pulp space by root canal treatment.
- dental pulp
- odontoblast
- tertiary dentine
- immune response
- carious lesion
- pulpitis
1. Introduction
Although small, teeth are complex structures composed of several components with unique architectural characteristics and functions (Figure 1). Crown and root are made of different mineralized tissues, namely enamel, dentine and cementum, encasing a soft tissue, the dental pulp. The root anchors the tooth to the surrounding bony tissue via short, tendon-like fibers called the periodontal ligament, which insert both into root cementum and bone.
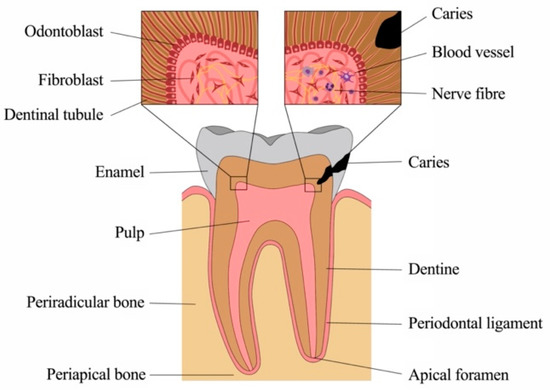
Teeth are prone to damage, mostly by caries, periodontal disease and trauma. In all these cases, microorganisms lead to infection and inflammation. Advanced methods and materials are available in dental medicine to date, where most therapies aim at the replacement of damaged or lost structures. Yet, oral tissues possess multiple means of pathogen recognition and defense, which are currently investigated and understood in increasing detail. An in-depth understanding of immune reactions of dental tissues, of cellular and molecular key players, as well as of temporospatial patterns of defense will enable approaches to improve diagnostics as well as treatment strategies, which can be more targeted, less invasive and aimed at tissue healing and regeneration rather than replacement.
1.1. Anatomy and Physiology of Sound Dental Tissues
1.1.1. Physiology of the Dentine-Pulp Complex
The craniofacial tissues are mainly derived from cells of the cranial neural crest. These cells develop in the dorsal region of the neural tube and then migrate into the 1st–fourth pharyngeal arch [1]. In the dental pulp, cranial neural crest-derived cells of the pulpal neurons play an important role in the regeneration of mesenchymal pulp cells and odontoblasts [2]. Most parts of the teeth are formed by cranial neural crest cells, namely dentine, cementum, periodontal ligament and the pulpal tissue, with the exemption of blood vessels and the enamel [3].
The dental pulp and the surrounding dentine (Figure 2A) form a unity, both developmentally and structurally. The pulp is made of mesenchymal soft connective tissue; it extends from a central chamber within the tooth crown into one or several root canals to the root apex. During tooth development and eruption, the presence of functional pulp tissue is a prerequisite for the completion of root formation. The pulp is lined with a layer of highly specialized cells, the odontoblasts (Figure 2B). These post-mitotic, polarized cells secrete a collagenous matrix, which later mineralizes to form dentine. This formative process occurs physiologically and continuously, not only during tooth development (primary dentine) but also later in life (secondary dentine). Each cell leaves a process behind, which becomes embedded in the mineralized tissue, giving dentine its tubular structure. While its composition is similar to that of bone, and odontoblasts share many characteristics with the osteoblasts, there are a number of distinct differences. Odontoblasts secrete dentine in a directional manner, and the cell bodies are not enclosed in the mineralized tissue. Thus, there is no physiological remodeling and replacement of dentine. Due to their origin from neural crest-derived ectomesenchyme and their unique localization, odontoblasts feature many more characteristics than just those of mineralizing cells. Shielded by enamel and dentine, they are the first line of cells to get in contact with toxins and compounds of oral bacteria once the mineralized matrices have started to break down, with caries being the most prevalent cause, followed by dental trauma. Thus, odontoblasts play a central role as mediators of both inflammation and repair processes [4][5][6][4,5,6]. Beneath the odontoblast layer and an adjacent cell-free zone, pulp fibroblasts populate an extracellular matrix mainly made of collagen type I and III [7]. The pulpal core is crossed by vascular and neuronal networks, which enter the tooth through the alveolar bone via the apical foramen. An accumulation of nerve fibers (plexus of Raschkow) can be found beneath the layer of odontoblasts; these follow the odontoblast processes into the dentinal tubules, making dentine and innervated tissue. It is assumed that there is either intimate crosstalk between the odontoblasts and the nerve fibers or that odontoblasts themselves participate in the transmission of external stimuli [8]. The majority of the pulpal tissue is composed of pulp fibroblasts. Stem cells, which are present in dental pulp in the perivascular niche, exhibit both mesenchymal and neural characteristics due to their origin from ectoderm. The pulp of healthy teeth is furthermore equipped with a variety of cells of the immune system. A recent study that investigated immune cells in healthy human pulp confirmed previous reports [9][10][9,10] and demonstrated that leukocytes (cluster of differentiation 45-positive/CD45+ cells) are present but contribute less than 1% to the total cell population [11]. Among these, granulocytes/neutrophils (CD16+ CD15+ CD14−) were found to be the major subpopulation, followed by CD3+ T lymphocytes, CD14+ monocytes and dendritic cells, whereas minor subpopulations included natural killer (NK) cells, B cells and regulatory T cells (Tregs) [11]. These immune cells, in correspondence with odontoblasts, pulp fibroblasts and pulpal stem cells, are essential for the initiation of immunological responses of the dental pulp to oral microorganisms.
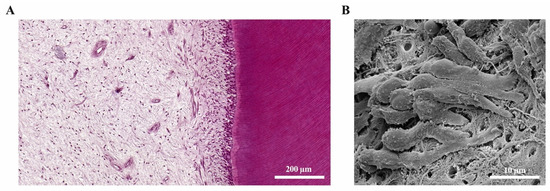
Figure 2. The dental pulp. (A) Histology of the dentine-pulp complex (own collection). (B) Odontoblast layer depicted by scanning electron microscopy (modified from [12]).
1.1.2. Physiology of the Apical Periodontium and Periradicular Tissues
Similar to the dental tissues, the skeleton of the face and a large majority of craniofacial connective tissue is derived exclusively from cells of the cranial neural crest [1][3][1,3]. Within the facial bones, in particular the alveolar bone, teeth are anchored via the periodontal ligament as part of the periodontium, which attaches the root cementum to the surrounding alveolar bone. The fibers of the periodontal ligament absorb and transmit forces between teeth and bone during the masticatory function. Alveolar bone undergoes constant physiologic remodeling in response to occlusal forces, which influence the number, density and alignment of trabeculae inside the bone.
Apical periodontitis is caused by an infection of the dental pulp that extends to the root pulp and leads to its necrosis [13]. The loss of the pulp tissue accounts for the loss of immune function, which allows microorganisms to access the alveolar bone via the root canal system. Periapical lesions can appear clinically as apical granulomas or radicular cysts and are both of inflammatory origin. In this context, the same etiology (necrosis of the pulp) leads to different clinical pathologies (apical granulomas and radicular cysts). Radicular cysts can grow to large extents and lead to the destruction of the periradicular periodontal tissue and the surrounding jaw bone [14]. In severe cases, even continuity resections of the mandible might be necessary for proper treatment of radicular cysts.
The epithelium of radicular cysts is most likely derived from the epithelial cell rests of Malassez (ERM). The ERM cells are a physiologic component of the periodontal ligament and originate from Hertwig’s epithelial root sheath (HERS), which governs dental root formation during embryologic development [15]. As the HERS undergoes an incomplete involution after the completion of root development, vital ERM cells remain in the periodontal ligament of the adult organism [15][16][15,16]. These ERM cells are critical for the physiology of periradicular tissues, as they keep their characteristics of epithelial cells despite the fact that they are embedded in a mesenchymal matrix. ERM cells are critical for periodontal ligament homeostasis and maintenance of the periodontal space, and they are involved in the prevention of ankylosis and root resorption [15].
2. Inflammatory Response of the Dental Pulp
Inflammatory reactions in the dental pulp have been described and extensively studied in response to a variety of insults such as caries, periodontal disease, operative procedures and dental trauma. As the oral cavity is heavily populated by microorganisms, the dental pulp is able to mount innate and later adaptive immune responses to inactivate and fight bacteria and their components that gain access during the carious process. In healthy teeth, a protective layer of enamel prevents the invasion of microorganisms through dentine and towards the pulp. Additionally, a continuous outward flow of dentinal fluid flushes out sporadically migrating bacteria. Carious demineralization of enamel caused by acidic metabolites of specific bacterial populations leads to disruption of the barrier, to cavitation and to degradation of dentine by Gram-positive bacteria, including streptococci, lactobacilli and actinomyces that largely dominate the microflora [17]. As both the diameter and the density of tubules that allow bacterial penetration increases with closer proximity to the pulp, this process accelerates with increasing depth of the lesion (Figure 3) [18].
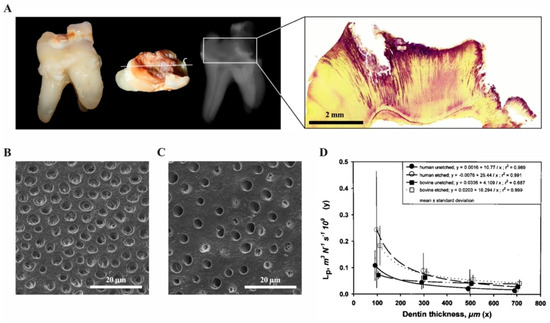
Figure 3. The carious process (own collection). (A) Extracted tooth with a deep carious lesion: clinical, radiographic and histologic appearance. The carious process has degraded enamel and dentine, which can be seen as radiolucent areas on the radiograph. After Brown and Brenn staining of the same tooth, bacteria are visible in the dentinal tubules (purple). (B) Dentine surface with dentinal tubules in a deep lesion close to the pulp and (C) a shallow cavity. (D) Permeability of dentine represented by hydraulic conductance Lp as a function of thickness for human and bovine dentin. Points with vertical lines represent the means and SD of the original data; lines represent the regressions of y vs. 1/x. (modified from [18]).
3. Inflammatory Responses in the Periapical Bone
Periapical lesions are considered an immunological defense reaction of the host to prevent the spread of bacterial infections from the root canal to the surrounding tissues [19][136]; most commonly, they present as apical granulomas (Figure 4). Most immune cells in periapical lesions are lymphocytes and macrophages [20][137]. Microbial components such as lipopolysaccharides get in contact with antigen-presenting cells (APC) like macrophages in the periapical tissue and induce the production of pro- or anti-inflammatory cytokines [19][136]. It is shown that proinflammatory cytokines like IL-1 and IL-6 can act as growth factors for ERM cells and may therefore promote radicular cyst formation [16].
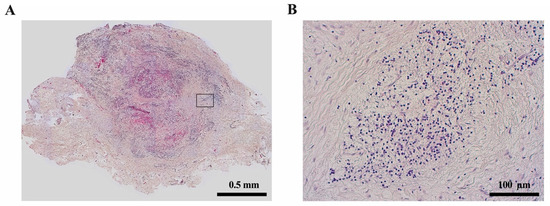
It is accepted that immunologic pathways contribute to the formation of radicular cysts in periapical lesions. However, the host defense processes in apical periodontitis are still a matter of research. In radicular cysts, macrophages showed a significantly higher degree of M1-like pro-inflammatory polarization compared to apical granulomas [21][138]. These cells might interact with ERM cells via cytokines and growth factors and promote their proliferation. In addition to macrophages, further immunological differences between radicular cysts and apical granulomas include the expression of human leukocyte antigen (HLA)-DR, CD83, macrophage colony-stimulating factor (MCSF) and Gal3, which appears to be significantly higher in radicular cysts than in apical granulomas [22][139]. The infiltration of CD4- and CD8-positive T cells and the CD4/CD8 ratio seems to not differ between apical granulomas and radicular cysts according to some studies [22][23][139,140], whereas others describe an increased CD8 infiltration in radicular cysts compared to apical granulomas [24][141].
These data indicate that the development of apical periodontitis towards apical granulomas or radicular cysts could be controlled immunologically, e.g., by changes in macrophage polarization. Radicular cyst formation was shown to be associated with an increased M1-like proinflammatory polarization of infiltrating macrophages [21][138]. Increased inflammatory activity may promote the formation of radicular cysts and increased bone resorption. Therefore, the use of root filling materials with anti-inflammatory properties like mineral trioxide aggregate (MTA) may counteract the development of radicular cysts and should be analyzed further in preclinical studies [22][139].
4. Resolution of Inflammatory Responses
4.1. Healing of the Dental Pulp
Although the immune response of the dental pulp exerts a variety of mechanisms to protect the soft connective tissues, extensive damage cannot be restored. Whereas proteases enable immune cells to passage through, they also dissolve the extracellular matrix, and immune cells not only harm invading microorganisms but also neighboring cells. Intense and prolonged stimulation results in chronic inflammation, premature aging and reduced defense mechanisms [25][26][54,96], or in tissue degradation, which enables bacteria to populate the root canal system and migrate into the periradicular tissues via the apical foramen.
Pathogen removal by therapeutic intervention can result in the resolution of inflammation, the elimination of remaining toxins, the secretion of anti-inflammatory signals and the production of tertiary dentine [6]. Apparently, the depth of the carious lesion is a critical factor, where a full host response is observed in lesions where the remaining dentine layer is less than 0.5 mm [27][142]. Furthermore, the progression rate plays a role, where rapidly spreading lesions are characterized not only by a different consistency and color but also by a differing microbiota [28][143]. In slowly progressing lesions, mineral deposition can detain invading bacteria and restrict tissue damage [6].
However, there is a close link between inflammation and repair, which was discovered with the observation that corticosteroids could compromise healing after myocardial infarction [29][144], and many proinflammatory mediators in pulpal inflammation can have differential effects [30][145], depending on their concentration. Compounds such as TGF-β and TNF-α, but also bacterial components can promote processes of repair at low concentrations, whereas they cause detrimental effects at higher levels. In addition, stem cell differentiation may be controlled by various proinflammatory mediators [31][27].
Not only the initial inflammatory response but also the reparative phase is characterized by the migration of various immune cells. In addition, nerve fiber sprouting beneath the site of injury [32][103] is guided by pulp fibroblasts by means of complement activation and secretion of brain-derived neurotrophic factor (BDNF), which enhances the outgrowth of neurites [33][52]. Other neurotrophic factors such as SP, VIP, CGRP and NPY may also play a role during regenerative processes as they promote angiogenesis and stimulate the deposition of tertiary dentine [34][35][146,147]. Both nerve growth factor (NGF) and BDNF are expressed in pulp cells; they have been suggested to enhance odontoblast differentiation and thus dentinogenesis [36][37][38][148,149,150].
Nevertheless, depending on the intensity of the stimulus and degree of damage to the pulp, repair and healing may not be possible, and chronic inflammation and eventually pulp necrosis may be the long-term consequence.
4.2. Healing of the Periradicular Bone
As microorganisms, which enter the periradicular bone via the apical foramen, cause an inflammatory response and subsequently a bony lesion, this process needs to be reverted in order to achieve healing. During endodontic treatment, antibacterial strategies aim at the elimination or at least drastic reduction of microorganisms within the root canal system in order to enable healing, which is then no longer hindered by infection. The process of healing begins with the inflammation and is resolved by the clearance of the immunogen/pathogen that induces the tissue response [39][151]. Thus, the integrity and regular function of the periradicular bone can be reestablished. Chronic or systemic conditions negatively affect the healing potential. Diabetes mellitus may be a modulating factor of endodontic infections and may compromise the healing process of periapical tissues [40][152]. Hyperglycemia elevates the levels of systemic inflammatory markers [41][153] and alters the various functions of the immune system [42][43][154,155]. In diabetic rats, the presence of endodontic infection and periapical lesions leads to increased numbers of neutrophils, lymphocytes and leukocytes, and the level of bone resorption in endodontic lesions was greater in diabetic compared to normoglycemic rats [44][156].
4.3. Stem cells in Repair and Regeneration
An important cell source during regular tissue turnover, but also during repair is the pool of resident stem cells within the dental pulp. Mesenchymal dental pulp stem cells can be harvested from permanent teeth [45][157] as well as deciduous teeth [46][158], furthermore from the apical papilla of immature teeth with incomplete root formation [47][159]. Stem cells in the dental pulp are located in the perivascular niche [48][160] and remain quiescent until they are recruited to the site of injury upon chemotactic signaling, they migrate and differentiate into a mineralizing cell type reminiscent of odontoblasts [49][161]. However, pulp stem cells also express TLRs and are capable of pathogen recognition [50][22], but may also be recruited after activation by macrophages [51][162].
Whereas carious lesions are the most common cause for inflammatory reactions, traumatic impact, for example, after crown fractures, may also expose the pulp to the oral cavity and thus enable microorganisms to access the pulp chamber. In the latter case, a healthy pulp can withstand bacterial invasion for several days. Animal studies in monkeys demonstrated that the inflammatory zone did not extend more than 2 mm into the pulpal tissue even after one week of exposure to the oral cavity [52][163], which highlights once more the remarkable ability of this tissue to withstand a bacterial attack.
