Studies describing invasive fungal infections (IFIs) after chimeric antigen receptor-modified T-cell (CAR-T-cell) therapy are limited. Although post-CAR-T-cell IFIs appear to be uncommon, they are associated with significant morbidity and mortality. Specific risk factors for IFIs in CAR-T-cell recipients have not been fully characterized and are often extrapolated from variables contributing to IFIs in patients with other hematologic malignancies or those undergoing hematopoietic cell transplant. Optimal prophylaxis strategies, including the use of yeast versus mold-active azoles, also remain ill-defined. Further research should investigate key risk factors for IFIs and establish an evidence-based approach to antifungal prophylaxis in these patients in order to improve clinical outcomes.
1. Introduction
Chimeric antigen receptor-modified T-cell (CAR-T-cell) therapy targeting the B-cell antigen CD19 has drastically improved outcomes in patients with refractory B-cell malignancies [1][2][3][4][5]. However, managing the toxicities of CAR-T-cell therapies remains challenging. The two most common of these toxicities are the cytokine release syndrome (CRS) and the immune effector cell-associated neurotoxicity syndrome (ICANS) (formerly known as CAR-T-cell associated encephalopathy syndrome). These toxicities typically develop within the first 21 days of CAR-T-cell infusion during proliferation of CAR-T-cells. Treatment of CRS and ICANS may include the interleukin-6 inhibitor tocilizumab and/or corticosteroids depending on their severity (graded 1–4) [2]. Prolonged leukopenia (particularly lymphopenia) and hypogammaglobulinemia due to B-cell aplasia are also two direct CAR-T-cell toxicities and are generally thought to be mediated by “on-target, off-tumor” effects of CAR-T-cells, which occur when CAR-T-cells kill normal B-cells that express the CAR-T-cell target antigen [6]. Neutropenia may also be a direct toxicity of CAR-T-cell therapy, but its pathogenesis has not been fully defined [6][7].
Chimeric antigen receptor-modified T-cell (CAR-T-cell) therapy targeting the B-cell antigen CD19 has drastically improved outcomes in patients with refractory B-cell malignancies [1,2,3,4,5]. However, managing the toxicities of CAR-T-cell therapies remains challenging. The two most common of these toxicities are the cytokine release syndrome (CRS) and the immune effector cell-associated neurotoxicity syndrome (ICANS) (formerly known as CAR-T-cell associated encephalopathy syndrome). These toxicities typically develop within the first 21 days of CAR-T-cell infusion during proliferation of CAR-T-cells. Treatment of CRS and ICANS may include the interleukin-6 inhibitor tocilizumab and/or corticosteroids depending on their severity (graded 1–4) [2]. Prolonged leukopenia (particularly lymphopenia) and hypogammaglobulinemia due to B-cell aplasia are also two direct CAR-T-cell toxicities and are generally thought to be mediated by “on-target, off-tumor” effects of CAR-T-cells, which occur when CAR-T-cells kill normal B-cells that express the CAR-T-cell target antigen [6]. Neutropenia may also be a direct toxicity of CAR-T-cell therapy, but its pathogenesis has not been fully defined [6,7].
Infections are among the indirect toxicities of CAR-T-cell therapy. CAR-T-cell recipients are at an increased risk of infection because of prior anti-neoplastic therapy, refractory malignancy, lymphodepleting conditioning chemotherapy (typically with fludarabine and cyclophosphamide), B-cell aplasia, the immune perturbations associated with CRS and ICANS, and their management with immunosuppressive therapies [8][9][10][11][12][13][14][15]. Nosocomial bacterial and respiratory viral infections are the most common infections after CAR-T-cell therapy. Invasive fungal infections (IFIs), in contrast, are uncommon, and studies providing detailed analyses of IFIs following CAR-T-cell therapy remain limited. Additionally, high-quality data informing antifungal prophylaxis practices are lacking.
Infections are among the indirect toxicities of CAR-T-cell therapy. CAR-T-cell recipients are at an increased risk of infection because of prior anti-neoplastic therapy, refractory malignancy, lymphodepleting conditioning chemotherapy (typically with fludarabine and cyclophosphamide), B-cell aplasia, the immune perturbations associated with CRS and ICANS, and their management with immunosuppressive therapies [8,9,10,11,12,13,14,15]. Nosocomial bacterial and respiratory viral infections are the most common infections after CAR-T-cell therapy. Invasive fungal infections (IFIs), in contrast, are uncommon, and studies providing detailed analyses of IFIs following CAR-T-cell therapy remain limited. Additionally, high-quality data informing antifungal prophylaxis practices are lacking.
2. Epidemiology of Fungal Infections after CAR-T-Cell Therapy
Seven published manuscripts and abstracts describing IFIs after CAR-T-cell therapy were identified at the time of this review [8][9][10][11][12][13][14]. Overall, IFIs after CAR-T-cell therapy are uncommon and have been reported in 1–15% of patients, with 0–10% and 0–7% of patients developing yeast and mold infections, respectively. Most IFIs occur within the first 30 days following CAR-T-cell therapy and typically represent breakthrough infections developing in patients receiving fluconazole or echinocandin prophylaxis. IFIs occurring >30 days after CAR-T-cell therapy, including invasive mold infections, have been described in patients with persistent risk factors such as prolonged neutropenia [8][9]. In one study which reported infections occurring >90 days after CAR-T-cell infusion, IFIs developed in 9% of patients and included two invasive mold infections, one yeast infection, and one endemic mycosis (
Seven published manuscripts and abstracts describing IFIs after CAR-T-cell therapy were identified at the time of this review [8,9,10,11,12,13,14]. Overall, IFIs after CAR-T-cell therapy are uncommon and have been reported in 1–15% of patients, with 0–10% and 0–7% of patients developing yeast and mold infections, respectively. Most IFIs occur within the first 30 days following CAR-T-cell therapy and typically represent breakthrough infections developing in patients receiving fluconazole or echinocandin prophylaxis. IFIs occurring >30 days after CAR-T-cell therapy, including invasive mold infections, have been described in patients with persistent risk factors such as prolonged neutropenia [8,9]. In one study which reported infections occurring >90 days after CAR-T-cell infusion, IFIs developed in 9% of patients and included two invasive mold infections, one yeast infection, and one endemic mycosis (Coccidioides immitis infection) [10]. Six studies reported infection-related deaths, of which mortality attributable to IFIs ranged from 0 to 5% [8][9][10][11][12][13].
infection) [10]. Six studies reported infection-related deaths, of which mortality attributable to IFIs ranged from 0 to 5% [8,9,10,11,12,13].
2.1. Yeast Infections
Fourteen yeast infections in 13 unique patients following CAR-T-cell therapy have been reported [8][9][10][11][14][15] (
Fourteen yeast infections in 13 unique patients following CAR-T-cell therapy have been reported [8,9,10,11,14,15] (). Seven of these episodes (50%) were cases of fungemia. Nine of the 14 yeast infections occurred within 30 days of CAR-T-cell infusion (early) including two Candida glabrata
fungemias; the remainder were fungemias caused by Candida tropicalis
, Candida krusei
, and Saccharomyces cerevisiae
. The additional early yeast infections described were two cases of respiratory tract infections attributed to C. glabrata
and Candida bracarensis
, one case of oropharyngeal candidiasis, and an intra-abdominal infection caused by C. glabrata
. Of note, as true Candida
respiratory tract infections are exceedingly uncommon in patients with hematological malignancies, the cases of Candida
respiratory infections may have simply represented colonization. All patients who developed early yeast infections were receiving fluconazole prophylaxis, with the exception of the patient who developed the S. cerevisiae
blood stream infection, who was receiving micafungin prophylaxis. Yeast infections >30 days after CAR-T-cell therapy were C. glabrata
fungemia, oropharyngeal candidiasis, Candida
esophagitis, and a case of Candida albicans
fungemia with subsequent vertebral osteomyelitis. Notably, these patients were not receiving antifungal prophylaxis, but no specific IFI risk factors were described in the studies. Infection-related mortality was attributed to the C. krusei
and C. tropicalis
fungemias, both of which were early infections.
Table 1.
Published reports of invasive yeast infections following chimeric antigen receptor-modified T-cell (CAR-T-cell) therapy. Neutropenia defined as absolute neutrophil count <500 cells/μL. Lymphopenia defined as absolute lymphocyte count <1000 cells/μL. ALL = acute lymphoblastic leukemia; DLBCL = diffuse large B-cell lymphoma; CRS = cytokine release syndrome; ICANS = immune effector cell-associated neurotoxicity syndrome. Dashes indicate that the data were not reported in the studies.
a
As true invasive
Candida
spp. respiratory tract infections are rare in patients with hematological malignancies, it is unclear if these isolates represent invasive infections or simply colonization.
| Ref. |
Fungal Infection |
Cancer |
Prophylaxis |
Neutropenia |
Lymphopenia |
Time of Onset of Infection |
CRS |
Steroids |
Tocilizumab Given? |
Previous
Transplant |
Died of Fungal Infection? |
| Park et al. [11] |
Saccharomyces cerevisiae | : fungemia |
ALL |
Micafungin |
Yes |
– |
Day 0–30 |
Grade 3 |
– |
– |
– |
No |
| Garner et al. [8] |
Candida tropicalis | : fungemia |
DLBCL |
Fluconazole |
Yes |
Yes |
Day 0–30 |
Grade 2 |
Yes |
Yes (2 doses) |
No |
Yes |
| Candida glabrata | : intra-abdominal infection |
DLBCL |
Fluconazole |
No |
Yes |
Day 0–30 |
Grade 1 |
Yes |
Yes (1 dose) |
Yes (autologous) |
No |
| Candida | esophagitis |
DLBCL |
Fluconazole |
No |
Yes |
Day 0–30 |
Grade 2 |
Yes |
Yes (1 dose) |
Yes (autologous) |
No |
| Candida albicans: | fungemia |
DLBCL |
None |
No |
Yes |
Day 30+ |
Grade 2 |
No |
Yes (1 dose) |
Yes (autologous) |
No |
| Candida albicans | : vertebral osteomyelitis |
DLBCL |
None |
– |
– |
Day 30+ |
Grade 2 |
No |
Yes (1 dose) |
Yes (autologous) |
No |
| Candida | esophagitis |
DLBCL |
None |
No |
Yes |
Day 30+ |
No |
No |
No |
No |
No |
| Hill et al. [9] |
Candida glabrata: | fungemia |
– |
Fluconazole |
– |
– |
Day 0–30 |
– |
– |
– |
– |
No |
| Candida glabrata: | fungemia |
– |
Fluconazole |
– |
– |
Day 0–30 |
– |
– |
– |
– |
No |
| Candida glabrata: | lungs | a |
– |
Fluconazole |
– |
– |
Day 0–30 |
– |
– |
– |
– |
No |
| Candida bracarensis: | lungs | a |
– |
Fluconazole |
– |
– |
Day 0–30 |
– |
– |
– |
– |
No |
| Tran et al. [15] |
Candida glabrata | : fungemia |
– |
– |
– |
– |
Day 30+ |
– |
– |
– |
– |
– |
| Cordeiro et al. [10] |
Oral candidiasis |
– |
– |
– |
– |
Day 30+ |
– |
– |
– |
– |
No |
| Louge et al. [14] |
Candida krusei | fungemia |
DLBCL |
Fluconazole |
– |
– |
38 days |
– |
Yes, for ICANS |
– |
No |
Yes |
2.2. Mold Infections
Of the 15 invasive mold infections (IMIs) described after CAR-T-cell therapy, 11, 3, and 1 were proven, probable, and possible IMIs, respectively [8][9][10][11][12][13][14]. Overall, the primary site of mold infection was the lung. Eight of the 15 IMIs occurred <30 days after CAR-T-cell infusion and included two
Of the 15 invasive mold infections (IMIs) described after CAR-T-cell therapy, 11, 3, and 1 were proven, probable, and possible IMIs, respectively [8,9,10,11,12,13,14]. Overall, the primary site of mold infection was the lung. Eight of the 15 IMIs occurred <30 days after CAR-T-cell infusion and included two Aspergillus
species (spp.) infections, two Mucorales infections, two Fusarium
spp. infections, an unidentified IMI, and one case of probable pulmonary aspergillosis. Of these early IMIs, 4, 3, and 1 patients were receiving fluconazole, micafungin, and voriconazole prophylaxis, respectively. The patient receiving voriconazole prophylaxis developed a Mucorales lung infection due to Cunninghamella
spp., but had previously been diagnosed with probable pulmonary mold infection (without a positive culture) prior to CAR-T-cell therapy; it was therefore unclear whether the Cunninghamella
infection was present prior to CAR-T-cell infusion, or whether it developed after therapy. Both Fusarium
spp. infections were disseminated. One involved the central nervous system, and one (caused by Fusarium solani
) was isolated from the patient’s thigh and sinuses; the latter infection developed while the patient was receiving fluconazole followed by posaconazole prophylaxis. IMIs occurring >30 days after CAR-T-cell therapy included three Aspergillus
spp. infections, one Mucorales infection, one case each of probable and possible invasive mold infection, and a skin and soft tissue infection from which both an Aspergillus
and Rhizopus
spp. were identified. Four of 15 (27%) patients who developed IMI died from their infection, three of whom died within 30 days of CAR-T-cell infusion. In one study describing three IMIs, two early infections occurred in patients who developed severe CRS/ICANS requiring tocilizumab +/− corticosteroids, and the single late IMI occurred in the setting of persistent disease and prolonged neutropenia [8]. Additionally, the central nervous system Fusarium
spp. infection occurred after the administration of a long course of steroids. Three of the studies reporting IMIs did not describe predisposing patient risk factors.
3. Anti-Fungal Prophylaxis Following CAR-T-Cell Therapy
3.1. Yeast Versus Mold-Active Prophylaxis
Because risk factors for IFIs in patients receiving CAR-T-cell therapy are not well-defined, there is no consensus about the optimal choice and duration of antifungal prophylaxis after CAR-T-cell therapy. As such, clinical practice varies widely among different centers. Although anti-yeast prophylaxis during the period of neutropenia after CAR-T-cell therapy has been the most commonly used strategy in clinical trials [3][4][5][9], it is not currently known whether certain subgroups of CAR-T-cell recipients may benefit from anti-mold prophylaxis. Indeed, there is much controversy around the optimal approach of yeast-versus-mold-active prophylaxis in these patients. Proposed strategies have included universal yeast-active prophylaxis, a tiered “risk stratification” approach, universal anti-mold prophylaxis, and pre-emptive therapy using fungal biomarkers and radiographic imaging [16][17][18]. At the center of the controversy is the absence of trials demonstrating whether anti-mold prophylaxis confers any mortality benefit in this population. Thus, robust evidence-based guidelines for antifungal prophylaxis such as those outlined by the European Conference on Infections in Leukemia [19] for other hematological malignancy patients do not currently exist.
Because risk factors for IFIs in patients receiving CAR-T-cell therapy are not well-defined, there is no consensus about the optimal choice and duration of antifungal prophylaxis after CAR-T-cell therapy. As such, clinical practice varies widely among different centers. Although anti-yeast prophylaxis during the period of neutropenia after CAR-T-cell therapy has been the most commonly used strategy in clinical trials [3,4,5,9], it is not currently known whether certain subgroups of CAR-T-cell recipients may benefit from anti-mold prophylaxis. Indeed, there is much controversy around the optimal approach of yeast-versus-mold-active prophylaxis in these patients. Proposed strategies have included universal yeast-active prophylaxis, a tiered “risk stratification” approach, universal anti-mold prophylaxis, and pre-emptive therapy using fungal biomarkers and radiographic imaging [25,26,27]. At the center of the controversy is the absence of trials demonstrating whether anti-mold prophylaxis confers any mortality benefit in this population. Thus, robust evidence-based guidelines for antifungal prophylaxis such as those outlined by the European Conference on Infections in Leukemia [28] for other hematological malignancy patients do not currently exist.
Nonetheless, in the past few years, several guidance documents have been published with provisional suggestions about the optimal approach to antifungal prophylaxis in these patients. Recent CAR-T-cell therapy expert panel guidelines suggest fluconazole or micafungin prophylaxis against Candida during neutropenia [1]. Another guideline from the European Society for Blood and Marrow Transplantation recommends mold-active azole prophylaxis in patients with prior allogenic HCT, prior invasive aspergillosis, and those receiving corticosteroids [20]. Other groups have suggested that ≥4 prior anti-tumor treatment lines, CAR-T-cell dose of >2 × 10
during neutropenia [1]. Another guideline from the European Society for Blood and Marrow Transplantation recommends mold-active azole prophylaxis in patients with prior allogenic HCT, prior invasive aspergillosis, and those receiving corticosteroids [29]. Other groups have suggested that ≥4 prior anti-tumor treatment lines, CAR-T-cell dose of >2 × 107/kg, prolonged neutropenia (≥3 weeks), and use of >1 dose of tocilizumab or the administration of other immunosuppressive agents (such as anakinra and siltuximab) for the management of CRS and ICANS should also warrant the use of mold-active antifungal prophylaxis [21][22].
/kg, prolonged neutropenia (≥3 weeks), and use of >1 dose of tocilizumab or the administration of other immunosuppressive agents (such as anakinra and siltuximab) for the management of CRS and ICANS should also warrant the use of mold-active antifungal prophylaxis [30,31].
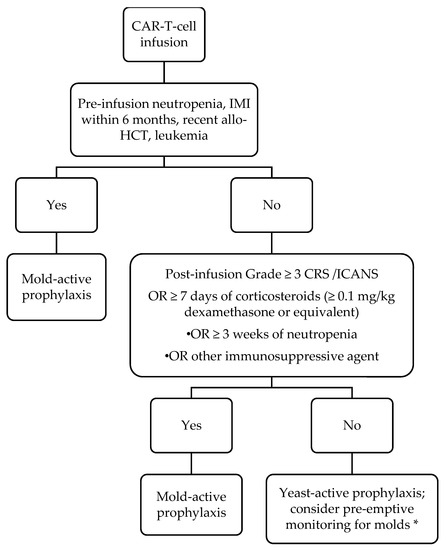
Based on the current literature and extrapolation from risk factors for mold infections in other hematological malignancy patients, we have adopted the antifungal prophylaxis protocol described in , which generally classifies patients as “high risk” for mold infection based on whether they are “AML-like” due to the presence of prolonged neutropenia, or “graft-versus-host disease (GVHD)-like” due to the use of corticosteroids or other immunosuppressive agents. We use posaconazole as our preferred mold-active prophylactic agent because of clinical trial data supporting its use in patients with AML and GVHD [23][24]. However, until data in CAR-T-cell therapy recipients are generated, we believe that any mold-active antifungal (such as voriconazole or isavuconazole) may be acceptable, and that the specific choice of agent should be guided by history of prior mold infection, side effect profile (e.g., avoidance of voriconazole in persons with neurotoxicity), and cost. Although this is our approach, others have advocated universal mold-active prophylaxis because of the uncertainties surrounding risk factors for mold infection after CAR-T-cell therapy. Specific concerns that were cited include the risks of mold infection in treatment-experienced ALL patients and challenges predicting duration of cytopenias and extent of steroid exposure [17].
, which generally classifies patients as “high risk” for mold infection based on whether they are “AML-like” due to the presence of prolonged neutropenia, or “graft-versus-host disease (GVHD)-like” due to the use of corticosteroids or other immunosuppressive agents. We use posaconazole as our preferred mold-active prophylactic agent because of clinical trial data supporting its use in patients with AML and GVHD [32,33]. However, until data in CAR-T-cell therapy recipients are generated, we believe that any mold-active antifungal (such as voriconazole or isavuconazole) may be acceptable, and that the specific choice of agent should be guided by history of prior mold infection, side effect profile (e.g., avoidance of voriconazole in persons with neurotoxicity), and cost. Although this is our approach, others have advocated universal mold-active prophylaxis because of the uncertainties surrounding risk factors for mold infection after CAR-T-cell therapy. Specific concerns that were cited include the risks of mold infection in treatment-experienced ALL patients and challenges predicting duration of cytopenias and extent of steroid exposure [26].
Figure 1.
Our approach to anti-fungal prophylaxis for prevention of invasive fungal infection post-chimeric antigen receptor-modified T-cell (CAR T-cell) therapy. Abbreviations: IMI = invasive mold infection; allo-HCT = allogeneic hematopoietic cell transplantation; CRS = cytokine release syndrome; ICANS = immune effector cell-associated neurotoxicity syndrome. For our purposes, we define neutropenia as an absolute neutrophil count of ≤500/µL. Duration of mold-active prophylaxis should be individualized. We maintain patients on mold-active agents until at least 1 month after discontinuation of immunosuppression AND resolution of neutropenia. Posaconazole is our preferred agent; voriconazole and isavuconazole are reasonable alternatives based on side effect profile and cost. * Pre-emptive therapy consists of diagnostics such as fungal biomarkers (serum beta-D-glucan, galactomannan) and surveillance radiographic imaging.
There are no data to guide the duration of mold-active prophylaxis. Although the paradigm in outlines a general framework for duration depending on the presence of neutropenia and the use of steroids, the precise duration of prophylaxis should be determined on a case-by-case basis based on the resolution of risk factors. A pre-emptive approach relying on biomarkers and imaging [25] has not been validated in these patients and may be hampered by limited testing availability and slow turnaround times. While the CD4
outlines a general framework for duration depending on the presence of neutropenia and the use of steroids, the precise duration of prophylaxis should be determined on a case-by-case basis based on the resolution of risk factors. A pre-emptive approach relying on biomarkers and imaging [34] has not been validated in these patients and may be hampered by limited testing availability and slow turnaround times. While the CD4+
T-cell cell count is an appealing marker that may help guide and individualize the duration of mold-active or other antifungal prophylaxis, further research validating this approach would need to be conducted prior to widespread inclusion of CD4+ T-cell. measurements in prophylactic algorithms. Ultimately, there is a need to conduct large multicenter prospective studies, preferably randomized clinical trials similar to the pivotal trials of posaconazole in AML and GVHD [23][24], to determine the benefit of yeast versus mold-active antifungal prophylaxis in CAR-T-cell therapy recipients.
T-cell. measurements in prophylactic algorithms. Ultimately, there is a need to conduct large multicenter prospective studies, preferably randomized clinical trials similar to the pivotal trials of posaconazole in AML and GVHD [32,33], to determine the benefit of yeast versus mold-active antifungal prophylaxis in CAR-T-cell therapy recipients.
3.2. Prophylaxis Against PCP
It is standard practice to administer trimethoprim-sulfamethoxazole (or alternatives, such as dapsone, atovaquone, and monthly intravenous pentamidine) for 3–6 months after CAR-T-cell therapy to prevent PCP [6][21][22]. Given that many CAR-T-cell patients are expected to experience prolonged lymphopenia due to “on-target, off-tumor” effects of CAR-T-cells, these patients may be at risk for PCP beyond 6 months. Some authors have suggested that PCP prophylaxis be continued until the CD4
It is standard practice to administer trimethoprim-sulfamethoxazole (or alternatives, such as dapsone, atovaquone, and monthly intravenous pentamidine) for 3–6 months after CAR-T-cell therapy to prevent PCP [6,30,31]. Given that many CAR-T-cell patients are expected to experience prolonged lymphopenia due to “on-target, off-tumor” effects of CAR-T-cells, these patients may be at risk for PCP beyond 6 months. Some authors have suggested that PCP prophylaxis be continued until the CD4+ T-cell count is greater than 200 cells/µL [21]. Indeed, cases of PCP have been reported over 6 months after CAR-T-cell therapy in lymphopenic patients whose PCP prophylaxis had been discontinued [8][9][13]. Based on these data, we currently recommend at least 1 year of anti-PCP prophylaxis at our center, which can be stopped once the CD4
T-cell count is greater than 200 cells/µL [30]. Indeed, cases of PCP have been reported over 6 months after CAR-T-cell therapy in lymphopenic patients whose PCP prophylaxis had been discontinued [8,9,13]. Based on these data, we currently recommend at least 1 year of anti-PCP prophylaxis at our center, which can be stopped once the CD4+
T-cell count is greater than 200 cells/µL.