- cirrhosis
- cardiomyopathy
- echocardiography
- treatment
- diagnosis
- pathophysiology
- Ejection Fraction
- Diastolic Heart Failure
- Cardiac MRI
- Speckled Tracking Echocardiography
1. Introduction
Table 1) of clinical features associated with cirrhotic cardiomyopathy, such as systolic dysfunction, seen as a blunting of the usual cardiac response to stress; diastolic dysfunction; and electrical dysfunction [3][4]. The technical definition [5] (
Table 1.
| Systolic Dysfunction | Diastolic Dysfunction | Supportive Criteria |
|---|---|---|
| Blunted increase in cardiac output on exercise, volume challenge or pharmacological stimuli | E/A ratio <1.0 (age-corrected) | Prolonged Q-Tc interval |
| Resting ejection fraction <55% | Prolonged deceleration time (<200 ms) | Enlarged left atrium |
| Prolonged isovolumetric relaxation time (<80 ms) | Increased myocardial mass | |
| Electrophysiological abnormalities | Increased BNP and pro-BNP | |
| Abnormal chronotropic response | Increased troponin I | |
| Electromechanical uncoupling/dys-synchrony |
Reported by Møller et al. [5].
Table 2.
| Systolic Dysfunction | Advanced Diastolic Dysfunction | Areas for Future Research Which Require Further Validation |
|---|---|---|
| Any of the following LV ejection fraction ≤50 % | ≥3 of the following | Abnormal chronotropic or inotropic response |
| Absolute GLS <18% or >22% | Septal é velocity <7 cm/s | Electrocardiographic changes |
| E/é ratio ≥15 | Electromechanical uncoupling | |
| LAVI >34 mL/m2 | Myocardial mass change | |
| TR velocity >2.8 m/s | Serum biomarkers | |
| Chamber enlargement | ||
| CMRI |
Modified from Izzy et al. [6].
2. Pathophysiology
Cirrhosis and portal hypertension lead to a hyperdynamic circulatory state, defined by an increase in total blood volume, a reduction in circulating volume due to splanchnic blood pooling, reduced systemic vascular resistance, and an increased cardiac output. In addition to these circulatory changes, there is direct injury to cardiac myocytes due to a combination of pro-inflammatory cytokines, elaboration of vasoactive peptides, and reduced response to sympathetic stimulation. A rat cirrhotic model has revealed that alterations in collagen configuration and titin modulation can lead to changes in diastolic function. [7] Early dynamic changes are largely driven by humoral responses. Splanchnic arterial vasodilation and a reduced hepatic capacity to metabolize vasoactive agents leads to a reduced effective arterial circulating volume and the activation of the central baroreceptors, leading to the activation of the sympathetic nervous system and the renin-angiotensin-aldosterone system (RAAS) (see ) [4]. This leads to the development of a hyperdynamic circulation, which is defined by an increased cardiac output, reduced arterial blood pressure, reduced vascular resistance, and increased heart rate [8].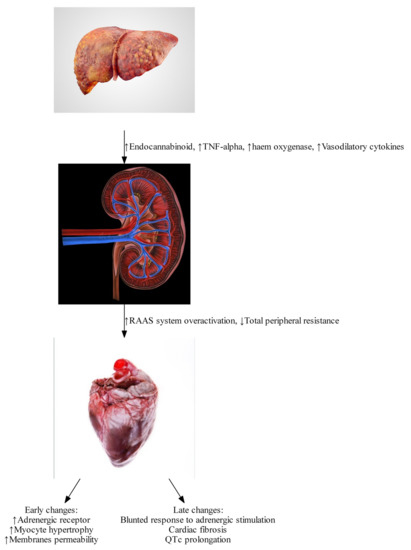
Figure 1.
https://www.istockphoto.com/photos/
2.1. Endocannabinoids
Endocannabinoids such as anandamide (AEA) and 2-arachidonoylglycerol (2-AG) bind to the cannabinoid-1 (CB-1) receptor. The endocannabinoid system is significantly up-regulated in the setting of cirrhotic cardiomyopathy [9][10] due to increased liver production of endocannabinoids, as well as increased expression of cannabinoid receptors. Activation of the endocannabinoid system can lead to reduced peripheral vascular resistance and increased splanchnic blood volume, worsening the effects of portal hypertension [11][12]. In animal models, improvements in peripheral vascular resistance, arterial blood pressure, and in cardiac
β
2.2. Tumor Necrosis Factor
α has been shown to induce the formation of endocannabinoids [10][12], and to promote a pro-inflammatory state, which further depresses cardiac function via activation of inducible nitric oxide synthase and elaboration of nitric oxide [13]. Nitric oxide has a direct negative inotropic effect, causing a cardio-depression similar to sepsis. This effect contributes to the early development of hyperdynamic circulation resulting from endeavors to compensate through other mechanisms. In a bile duct ligated mouse model, use of inducible nitric oxide inhibitors has been shown to reverse cardiac depression [13].
2.3. Haem Oxygenase
2.4. Bile Acid
Animal models have demonstrated the negative inotropic effect of elevated bile acid levels [17]. Elevated bile acid levels cause cardiac arrhythmia and disruption of channels, leading to cardiotoxicity [17][18] Elevated bile acids are thought to have cardiotoxic effects in human hearts, although ursodeoxycholic acid has been shown to have a cardioprotective effect [19]. There is a need for more in vitro studies before the effects of bile acids on human cardiac system can be fully comprehended.
2.5. β Adrenergic Responsiveness
β
β
2.6. Myocardial Fibrosis and Myocyte Hypertrophy
β
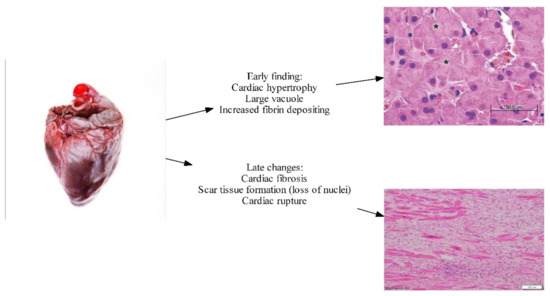
Figure 2.
2.7. Altered Cardiomyocyte Membrane Fluidity and Ion Channel Defects
2+
+
+
Results from animal models of cirrhotic cardiomyopathy have shown a diminished capacity for ion transport across the cell membrane [28][29]. These changes contribute to both impaired cardiac contractility and electrophysiological abnormalities [21], including prolonged QTc. An increase in membrane cholesterol content has been associated with these changes, although the underlying mechanism is unclear.
3. Treatment of CCM
Studies on CCM treatment are extremely limited. This may be largely because CCM was recognized as a temporary phenomenon that could be corrected with a definite treatment plan of liver transplantation [30]. However, studies emerging within recent decades indicate a paradigm shift as CCM is being recognized as a major co-morbidity that requires expert evaluation with specific treatment end goals that must to be met to improve morbidity and mortality outcomes [31].
Studies on CCM treatment are extremely limited. This may be largely because CCM was recognized as a temporary phenomenon that could be corrected with a definite treatment plan of liver transplantation [37]. However, studies emerging within recent decades indicate a paradigm shift as CCM is being recognized as a major co-morbidity that requires expert evaluation with specific treatment end goals that must to be met to improve morbidity and mortality outcomes [38].Treatment for cirrhotic cardiomyopathy is an area of ongoing research and includes a number of challenges. Patients recruited to clinical trials tend to have less severe cirrhosis [32]; however, most patients diagnosed with cirrhotic cardiomyopathy will have advanced cirrhosis. This creates a dilemma of whether treatment outcomes could be extrapolated and applied to other patients with CCM. Despite this challenge, investigation into the basic mechanisms of cirrhotic cardiomyopathy has uncovered some potential treatment targets, including the pro-inflammatory system and the endocannabinoid system [33]. Other treatments under investigation include traditional heart failure medications, with some trials showing positive results [34].
Treatment for cirrhotic cardiomyopathy is an area of ongoing research and includes a number of challenges. Patients recruited to clinical trials tend to have less severe cirrhosis [34]; however, most patients diagnosed with cirrhotic cardiomyopathy will have advanced cirrhosis. This creates a dilemma of whether treatment outcomes could be extrapolated and applied to other patients with CCM. Despite this challenge, investigation into the basic mechanisms of cirrhotic cardiomyopathy has uncovered some potential treatment targets, including the pro-inflammatory system and the endocannabinoid system [39]. Other treatments under investigation include traditional heart failure medications, with some trials showing positive results [40].3.1. β Blockade
β blockers are the standard of care for many forms of cardiac failure, and their use improves mortality in heart failure with reduced ejection fraction [35]. However, cirrhosis is often an exclusion criterion in these trials. In patients with cirrhosis,
β-blockers have been studied in the treatment of portal hypertension [36]. Randomized controlled trials of metoprolol and carvedilol in patients with cirrhotic cardiomyopathy showed no significant differences in mortality, hospitalizations, or cardiac function [30]. It was postulated that negative results may have been due to inadequate heart rate suppression in these patients [30].
A trial performed by Silvestri et al. [30] managed to suppress the heart rate to less than 65 beats per minute, which is the standard for other clinical trials in cardiology. Despite this, the trial failed to show any differences in mortality, hospitalizations, or cardiac function [30]. This trial was limited to a small sample size, and lacked variation in
β blockers being tested, limiting the possible inferences and increasing the likelihood of type II errors [30].
3.2. Ivabradine
Inward sodium potassium channel within sinoatrial node
If
‘funny channel’) inhibitor, has been shown to improve mortality in combination with beta blockers when used in patients with reduced ejection fraction [37]. The combination of ivabradine and carvedilol improves mortality in patients with cirrhotic cardiomyopathy with diastolic dysfunction compared with standard care [34]. This was demonstrated by Premkumar et al. [34] in a trial where a combination of low dose ivabradine and carvedilol were compared head-to-head with standard cirrhotic liver care. There was an improvement in mortality and a reduction in liver cirrhosis-related events in patients who were able to achieve a heart rate of 55 to 65 beats per minute.
Despite this positive outcome, it is important to recognize that these are early studies of the application of anti-congestive cardiac failure medication in CCM patients. The dose utilized by the Premkumar et al. [34] trial was low, with none of the known potential side effects, such as atrial fibrillation due to ivabradine, observed in patients in the treatment arm. One interpretation of these findings is that the boundary was perhaps not pushed hard enough out of an overabundance of caution. It will be critical to conduct more extensive studies, particularly on dosing, before
β
3.3. Liver Transplantation
Patients with cirrhotic cardiomyopathy face significant risk in the pre-operative, intraoperative, and peri-operative period [38]. Liver transplantation has been thought of as the definitive intervention for cirrhotic cardiomyopathy [4][39]. Self-control studies have shown a significant reversal of cardiac changes post-transplant [38]. There is hope that the underlying process leading to improvement can be elucidated, and lead to future targeted therapies [4]. Following transplantation, there are improvements in QTc, exercise tolerance, echocardiography parameters, and biochemistry such as troponin and BNP. A summary of the issues for this discussion is shown in

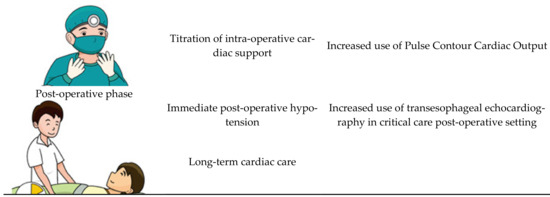
Figure 34.
While it remains true that transplantation will reverse the majority of the pathology, some pathological processes may remain active beyond the peri-transplant period. For that reason, it is now routine practice for the screening of CCM to be done in all liver transplant candidates. Screening methods involve traditional transthoracic echocardiography or stress echocardiography. The application of transthoracic echocardiography in the paediatric population has been shown to significantly improve care by identifying high risk patients prior to transplantation [40]. Less favorable transthoracic echocardiography parameters are associated with a prolonged ICU length of stay in the post-transplant period, making it an invaluable pre-operative assessment tool [41]. We suggest that cardiac MRI should also be routinely performed where possible, given that it provides valuable information regarding cardiac function as well as for risk-stratifying the patient [42].
During the intra-operative state, patients with CCM have a much higher adverse outcome due to complex fluid status as well as significant negative inotropic effects of general anesthesia on a cardiovascular system already under stress [43]. Most transplant cases last for hours, and data is emerging which shows that, for patients with CCM, invasive blood pressure monitoring does not truly reflect perfusion [44]. There is thus a growing consensus that transesophageal echocardiography should be performed to help titrate inotropic support intra-operatively [45]. The application of echocardiography intra-operatively, however, is currently limited to specialized institutions, given that not all anaesthetists are trained in evaluating the cardiac output on invasive echocardiography. There is emerging evidence that comprehensive cardiac output assessment, such as pulse contour cardiac output, should be standard of care in all liver transplantations [43].
During the post-operative period, there are major changes in vascular resistance along with portal venous system that generate major stresses on the cardiovascular system [43]. These patients already face a challenging transplantation journey from a significant pre-transplant disease burden [46]. Unsurprisingly, a significant number of cardiac complications has been observed in transplant patients, with the most common being refractory hypotension [47]. The current literature suggests that transesophageal echocardiography should be done immediately post-transplant as standard of care, because it provides invaluable information relevant to the required level of post-transplant cardiac support [41] (see
Figure 3).
