Tunneling nanotubes (TNTs) are recognized long membrane nanotubes connecting distance cells. In the last decade, growing evidence has shown that these subcellular structures mediate the specific transfer of cellular materials, pathogens, and electrical signals between cells. As intercellular bridges, they play a unique role in embryonic development, collective cell migration, injured cell recovery, cancer treatment resistance, and pathogen propagation. Although TNTs have been considered as potential drug targets for treatment, there is still a long way to go to translate the research findings into clinical practice.
- tunneling nanotubes
- intercellular communication
- heterogeneity
- communication efficiency
1. Introduction
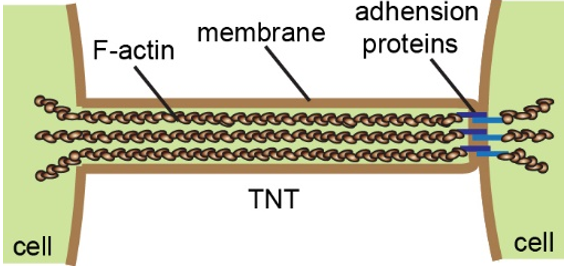
Multicellular organisms coordinate cell behavior, regulate morphogenesis, and maintain tissue homeostasis by secreting chemical molecules, releasing exosomes, and establishing direct connections such as neuronal synapses and gap junctions [1]. In 2004, the group of Gerdes reported for the first time a new way of long-distance cell–cell communication, tunneling nanotubes (TNTs) [2]. TNTs are tubular membrane structures with diameters of several hundred nanometers, which contain F-actin and, usually, adhesion proteins at one end (Figure 1) [3][4][3,4]. Hovering above the substrate, they directly connect adjacent cells up to hundreds of micrometers apart. These unique morphological features make them different from other cellular protrusions, such as filopodia and cytoneme. Numerous studies over the past ten years have shown that TNTs are widely present in various cell types [5][6][5,6]. More importantly, TNTs enable the transfer of small molecules, proteins, vesicles, and organelles between cells [2][7][8][9][10][11][2,7–11]. In 2010, we discovered that TNTs mediated depolarization coupling in non-neuronal cells, indicating that TNTs facilitate electrical signal transduction in addition to material transport [12]. Indeed, due to the characteristics of long-distance, high specificity, and multilevel transportation, TNT communication was dubbed the Internet of cells [13].
Figure 1. The schematic representation illustrates the structure and general composition of tunneling nanotubes (TNTs).
2. TNTs Are Heterogeneous Structures
2.1. Difference in Morphology
TNTs exhibit high variability in their morphology in terms of length and thickness [5][14][5,25]. Though the length of TNTs usually changes with the cell spacing caused by cell movement, it ranges from 10 to 100 µm in most types of cells. In a few cases, TNTs have been described as long cytoplasmic extensions up to 300 µm in length [6]. However, these fragile structures may break during prolongation if the pulling force by the cells exceeds the mechanical strength of TNTs [2][15][2,26]. In any event, the maximum length of a TNT is crucial since it determines the communication distance between cells. By analyzing the electron microscope images, the diameter of the TNTs was measured from hundreds of nanometers to a microscale [16][27]. One explanation for such a variation is that TNTs containing microtubules display thicker morphology [17][18][19][19,28,29]. Using cryo-electron microscopy, Sartori-Rupp and his colleagues recently revealed that TNTs were composed of a bunch of ultrathin tubes in mouse catecholaminergic CAD cells and human neuroblastoma SH-SY5Y cells, which are hardly distinguished by conventional confocal microscopy [3]. According to this study, the diameter of a TNT lacking microtubules may be determined by the number of ultrathin tubes. Since the identification of TNTs is still based on their morphological characteristics, the morphological diversity of TNTs has brought confusion to the nomenclature and the literature review on TNT research. The establishment of criteria for the classification of TNTs will be of great significance in this field.
2.2. Different Mechanisms of TNTs Formation
The importance of F-actin in TNT formation was first proved by our early study showing that a low dose of cytochalasin B, an actin inhibitor, could reduce the number of TNTs in PC12 cells [20][34]. Even in the MT-TNTs, F-actin plays a dominant role as well, because the treatment of microtubule inhibitors did not significantly disrupt the TNT structures [17][21][19,35]. Due to this line of thought, researchers considered that actin regulators and motor proteins were implicated in the formation of TNTs. Many laboratories successively identified several key proteins and signal pathways regulating TNT formation in different types of cells, such as M-sec/ERp29 [22][23][24][36–38], p53/Akt/PI3K/mTOR [25][26][27][39–41], Myosin10 [28][29][42,43], CDC42/IRSp53/VASP [30][44], and Rab11a/Rab8a [31][32][45,46]. Paradoxically, TNTs were also observed in M-sec or p53-deficient cells [28][33][42,47]. Such inconsistent results imply that the biogenesis of TNTs may not have a universal molecular regulation mechanism, probably due to their heterogeneity [34][30].
2.3. Where and When Do TNTs Form?
Although the evolutionary significance of TNTs biogenesis is completely unknown, cells under specific physiological circumstances may need these unique structures to establish communication that could not be accomplished by other types of intercellular connections: (i) distant cells connection [4][35][36][4,57,58], (ii) cells migration or invasion [37][38][39][20,59,60], and (iii) heterogeneous cells interaction [40][41][42][43][17,61–63]. Interestingly, the discovery of TNT-like structures between bacteria may provide clues in the study of the putative evolution of TNTs from bacteria to mammals [44][64]. Whether there are extracellular signals that trigger the formation and directionality of TNTs is still an exciting question. At least, quite a lot of studies have described that the number of TNT-like structures increased in inflammatory and stress conditions, such as pathogen infections [45][46][47][21,31,65], oxidative stress [48][66], high intracellular calcium concentrations [22][36], inflammatory signals [49][50][67,68], misfolded proteins, and pathogenic amyloid aggregates [8][28][51][52][8,42,69,70]. Moreover, the tumor microenvironment (hypoxia, acidic pH, hyperglycemia, and serum deprivation), as well as chemo- and radiotherapy-induced reactive oxygen species (ROS) production, leads to more TNTs in tumor cells [25][48][53][54][55][56][57][58][39,66,71–76]. Additionally, exosomes derived from malignant cells or vesicle recycling induce an increased rate in the formation of TNTs [32][59][46,77]. Under these circumstances, cells may respond to the stresses or stimulations by activating signaling pathways that initiate cytoskeleton rearrangement and cell movement, which consequently promote the formation of TNTs.