PCarotons (H+) are highly reactive, they are always solvated or disolvated. In presence of water aboxylic acids dissociate in an exothermic reaction into the acid-anion and water to carboxylates (R-COO-) and oxonium (H+[H2O]n.n) ions. The Proton tTransport cChain (PTC) hypothesis was developed forasserts that enzyme- complexes. The assumption that the enzyme-enzyme interaction is bridge nascent acids and ensure water-free entails that an acid synthesized from enzyme A is transferred as acid to enzyme Btransfer of the intermediate substrate. The PTC- hypothesis was first discussed for the GAPDH-LDHm complex. GAPDH formed NADH-H+ is transferred to LDHm. The consequence of water-freeentails that the concentration of the transfer is that the concentration of NADH-H+ is irred acid is mathematically infinite. A and an infinite concentration drives enzymatic reactions unidirectionally drives the LDHm catalysed reaction. LDHm activity strictly depends on delivery (mol/s) of NADH-H+. The PTC hypothesis replaces the well-established. In support of this, a number of enzymes, such as proton-linked monocarboxylate transporters (MCTs), lactate dehydrogenases (LDHs) or sodium/hydrogen exchangers have been experimentally determined to catalyse unidirectionally. In addition, enzyme complexes, such as the pyruvate dehydrogenase complex (PDHc), are also known to catalyse unidirectionally. Scientific concept of (single) enzyme-kinetics by enzyme-complex kinetics. Quite well-known proteins complexes driven by PTC are: the pyruvatedehydrogenase complex (PDHc) or the citric acid cycles, such as the original Citric Acid Cycle proposed that acids are metabolized in a clockwise direction. The PTC hypothesis provides mechanisms, mathematics and law of nature for biological processes.
- PTC
- Citric Acid Cycle
- PDHc
- LDH
- GAPDH
- MCT
Background
Protons (H+) are completely removanished from didactic biochemistry. A century ago, O. F. Meyerhof set glycolysis as the degradation of glucose (C6H12O6) to two molecules lactic acid (2 (x C3H6O3) [1]. Today, the stoichiometry in tohe metabolize sm of one molecule of glucose to two lmolecules of lactic acid is generally unknown (lacH) has vanished from common understanding. In 1953, H. A. Krebs was honoured with the Noble Prize for the discovery of the cCitric acid cAcid Cycle. Krebs presented lactic acid aH as substrate of a cycle unidirectionally cycling accarboxylic acids [2]. Today, the acid-anioncarboxylate pyruvate wa(pyr-) is considered as the substrate of a “Krebs “citrate cycle”. The change from KrebsMeyerhof’s and Krebs’ scientifically based modelconcepts to an alchemistic citrate cyclemodel of glucose metabolism started quite early soon after their discoveries. In 1949 Kennedy and Lehninger investigated mitochondrial function on isolated mitochondria [3]. Kennedy and Lehninger usedThey used the term carboxylates and determined carboxylates such as, pyruvate-, malate, a-ketoglutarate and citrate. They discussed their data on basis of Krebs’ cCitric acid cAcid Cycle, but phrases such as: “.…oxidation of pyruvate and other intermediates of the Krebs cycle.” and “…aerobic citrate formation from pyruvate when malate served as a source of oxaloacetate.” misquoted Krebs’ acid cycle. But, to say misquote is incorrect, because Kennedy and Lehninger missfailed to quocite one Krebs’ manuscript of Krebs.ts. Yet somehow, Lehningers “’ models of ‘citrate cycle” hass’ and ‘glycolysis’ have conquered the world.
KDuring formulation of the PTC hypothesis, the knowledge of glucose metabolism was reset to the original work [4].
Enzyme complexes driven by PTCs
PTCs have been inedy andvestigated for decades. Unfortunately, by not adding H+ into Lthehninger also ment chemical formula and giving enzymes incorrect names, such as pyruvate dehydrogenase or pyruvate carboxylase, the concept of PTCs was largely hidden from the mainstream.
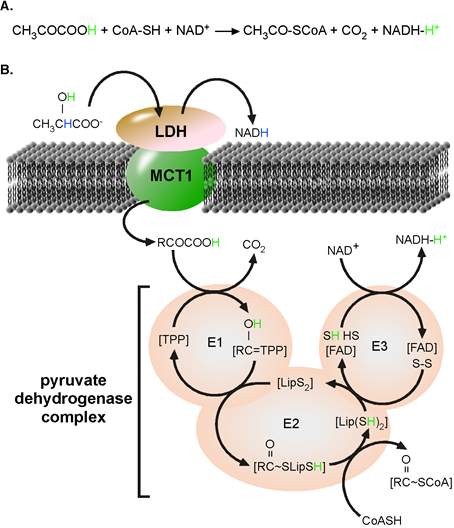
Pyruvate dehydrogenase complex (PDHc): PDHc together with a-ketoneglutarate dehydrogenase belongs to the family of a-ketoacid dehydrogenases [5]. Pyruvic acid (pyrH) is “Fluoride was the substrate of PDHc and proton-linked MCT1 is one transporter catalysing the transfer of pyr- and H+, pyrH to PDHc. The functional proton-linked MCT1•hed to inhibit enolase and the end-point measured manometrically indicated tart LDH (LDH-h) complex is located at the inner mitochondrial membrane [6]. The kinetics and reaction catalysed the proton-linked MCT has been investigated and published many times [7]. Similarly, PDHc catalyses a well-known chemical reaction [8]. In form of an acid, the a-keto group of pyrH is partially positively charged allowing a nucleophile substitution. The net reaction and mechanism of the unidirectional acting PDHc is depicted in Figure 1.
Figure 1. PDHc complex catalysed reaction. (A) Chemical formation of 3-phoshoglyceric acid, which causes CO2ula for the overall reaction. (B) Mechanism of reaction catalyzed by the pyruvate dehydrogenase complex. LDH catalyzes the oxidation lactate to pyruvate. Nicotinamide adenine dinucleotide (NAD+) is reduced to NADH and a reactive H+, originally from the α-hydroxyl group of lactate (green). In the absence of water, pyruvate disolvates the libneratiw reactive H+ ton from bicarbonate”. In Lehningers texorm pyruvic acid. Monocarboxylate transporter 1 (MCT1) promotes the charge-neutral membrane transfer of pyruvic acid. Within the PDH complex, H+ from tbhe α-hydroxyl grooks 3-phoshoglyceric acid as product of phosphoglyceratup of lactate (green) takes active part in all steps of the reaction and finally ends disolvated by NADH. E1, pyruvate dehydrogenase; E2, dihydrolipoamide acetyltransferase; E3, dihydrolipoamide dehydrogenase; FAD, flavin adenine dinucleotide; Lip, Lipoyl; TPP, thiamine pyrophosphate. Image adapted from Roosterman et al. 2018 [4].
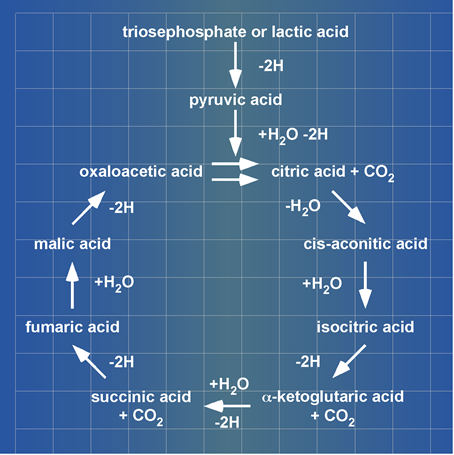
The Citric Acid Cycle enzyme complex: H. A. Krebs kinase (PGK) catalysed reaction is presented at his Noble Prize lecture lactic acid (lacH) as substrate and carboxylic acids being unidirectionally (clockwise) cycled (Figure 2).
Figure 2. A blueprint of Krebs’ Citric Acid Cycle. Krebs presented thimplified to 3-ps cycle of carboxylic acids at his Noble Prize lecture in 1953. ©The Nosbel Foundation [2].
Proton-linked MCT1•carbonic anhydrase II (CAII) enzyme complex: Prohtoglyceratn-linked MCT1 has been characterized as a unidirectional importe
Tr of monocarboxylic acids and the PTC hypothesis providiscussed plasma membes the mechanism and rationale for this unidirectional transport [9]. The proton-linked MCT1•CAII complex functionally links the energy of permanently emitted carbonic acid (H2CO3) (the end product of oxidane located PGK intive phosphorylation) with the import of the substrate of oxidative phosphorylation, lacH [4]. Thus, the complex withacts as a carboxylic acid/carbonic acid antiporter.
Proton-linked MCT4•phosphoglycerate kinase complex: pProton-linked MCT4 is also a unidirectional transporter, but acts as an exporter of monocarboxylatic acids [10]. Similarly, here, the transfer of the energy entity H+, initiates the monocarboxylic acid transfer. As yet the definitive prorter 4 (MCT4)ton-donor enzyme producing the acid is unknown. However, the proton-donor enzyme must be located at the plasma membrane for direct (water-free) H+-transfer. PAn increase in thermanent delivery of rate of glycolysis (metabolism of glucose to two molecules of lacH) is linked with an increased export of lacH, indicating that at least one product of the glycolytic enzymes has to be an acid. The development of the PTC hypothesis provoked reconsideration of not only the well-established chemical formula of glycolytic enzymes, but also the subcellular location of glycolytic enzymes. Intriguingly, phosphoglycerate kinase (PGK) can be located at the plasma membrane and the product of the PGK catalysed reaction is 3-phosphoglyceric acid drives MCT4 to [4]. Thus, a proton-linked MCT4•PGK complex was postulated to link the unidirectionallyl export of monocarboxylic acids [4] [5] [6] [7]with the rate of glycolysis rate.
P
Glyceraldehyde-3 phosphate dehydrogenase•muscle LDH complex: The unidirectional nasma membrane ture of this enzyme complex was discussed in the PTC hypothesis. The enzyme complex always catalyses the unidirectional transfer of NADH-H+ from GAPDH to muscle LDH (LDH-m) and reduces pyr- to lacatetate (lac-) [11] [4]. Thus, lac- is always a product Mof glycolysis.
Proton Transport Chains provide an alternative view on biological processes
The PTCT1 hypothesis is discussquestions and replaces most of what is considered to be well established in cbiochemistry:
Mathematics: The mathematics of enzymplex with carbonic anhydrase II (CAIIe kinetics understands enzymes to reversibly catalyse an equilibrium depending on concentration [mol/L]. The deduced mathematics of enzyme complex kinetics understands enzyme complexes to catalyse unidirectionally depending on the provision of the acid (mol/s).
Laws of nature: PMaximal ermanent export of carbonic acid (H2CO3)ntropy, or random distribution and movement of enzymes, substrates and products are the premise to apply the mathematics of enzyme kinetics [12]. The PTC hypothesis is based on the diametrical opposite of maximal entropy; idrives MCT1 to unideal organized systems. The PTC hypothesis inspired the transfer of the 4th law of thermodynamics frectionalom physics to biological processes [13]. The 4th law asserts that the flow of energy import monocarboxyl and material is sufficient to form ordered structures [14]. Whereas maximal entropy ignores ordered structures such as enzyme complexes, metabolons, organelles, membranes, cells, organism, biology in general; the 4th law inc acids [4] [5] [6] [7]orporates these and provides a path to self-organization.
M
Definitions of glucose metabolism: Glycolysis was defined as cytochondsolic process, starting with hexokinase II as first enzyme and pyr- as final membrane located LDHh-MCT1 was discussed to catalyse the first reaction of Krebs citric acid cycle: oxidation of lactate to pyruvate anproduct. This definition was replaced by considering glycolysis as a flow of energy and material (mol/s). Glucose transporters (GLUTs) are set as first enzymes and proton-linked MCT4•PGK as final enzyme complex. The definition that glucose metabolism provides metabolites to be “burned” and used as building blocks was expanded by setting glucose metabolism as the operating system of the cell and ‘major in command’. Metabolites are not simply intermediates but are defined as signalling molecules. Glucose and its metabolites not only promote the release of insulin, but also the release of a variety of cytokines, interleukins and growth factors [15]. Thus, hormones and transmitters are set as ‘second in command’. Unidirectionally importing or exporting proton-linked MCT complexes are classified as metabolic receiver- or sender-complexes, respectively [16]. Glycogen stores are considered not only as stores of energy, but also as stores of metabolic signalling molecules.
History of deleting protons in biochemistry
As mentioned above membrane transfer of pyruvic acid to the PDHc complexisquoting pioneering work on glucose metabolism and consequently refusing to integrate scientific progress in a didactic model of glucose metabolism has a long history. The huge variety of ‘Krebs’ Citrate Cycles’ propagated in textbooks and learned by rota have nothing in common with Krebs’ scientifically based concept of a Citric Acid Cycle.
M The high similaritoy between Kennedy and Lehninger’s chondrial membrane located LDHh-MCT1 was also introduced to provide anges of Krebs’ work and the actually propagated models of “Krebs’ citrate cycles” strongly suggests that Krebs’ work belongs to the most often quoted, but never read and never referred to publications in history [3]. The 70-year-old original Citric Acid Cycle merged the PDHc-catalysed formation of acetyl-SCoA and the citrate synthase-catalysed reaction into one step. Krebs formulated the reaction as follows (see Figure 2): pyruvic acid t + oxaloacetic acid + water ® citric acid + 2H + CO2. A. Lehninger’s textbook Biochemistry also mentioned this point, but detailing the reaction as: pyruvate car+ oxaloacetate ® citrate + CO2. [12].
It must be mentioxylase as substrate of oxaloacetic acid synthesis [4] [5] [6] [7].
Tned, that both Krebs and Meyerhof experimentally determined carboxylates and both knew that acids dissociate in water and enzymes act reversibly, but interestingly both created concepts with carboxylic acids and unidirectionally acting enzymes. The key to their scientifically based concepts, which is different to the experimentally gained data, is applying scientific rules, such as the principle oposed citrf mass conservation or stoichiometry.
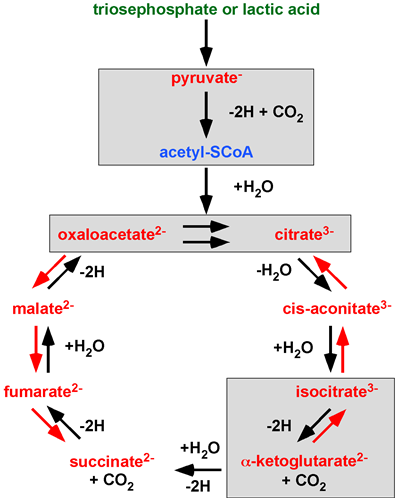
Actually, every single c acid cycle 1.1 balances burning of lactic acid and malic acid synhemical reaction published by H. A. Krebs is incorrectly transferred when a ‘Krebs’ citrate cycle’ is introduced. Krebs aimed to investigate and understand the scientific field of metabolism. All of Krebs’ work misunderstood by A. Lehninger is highlighted in Figure 3. The PTC hypothesis works with the original concept of the Citric Acid Cycle, a unidirectional cycle of carboxylic acids. The PTC hypothesis, by providing the missing rationale (enzyme complexes and water-free transfer), mathematics (infinite concentration) and the law of nature the PTC hypothesis. gives Krebs’ Citric acid cycle 1Acid Cycle a second chance.
Figure 3.1 A ‘Krebs’ citrate cycle’ variant found in textbooks, scientific publications and metabolic databanks. 70 pyearovides the mechanism of ts of deletions (green), additions (blue) and integrated changes (red) to Krebs’ Citric Acid Cycle. Chemical reactions showing alchemy or incorrect stoichiometry are highlighted in grey boxes. Image adapted from Krebs’ Noble Prize lecture in 1953. ©The Nobel Foundation.
Lehninger has O.changed the work of O. F. Meyerhof presented at his Noble Prize lecture: up-taken lacticand H. A. Krebs and the work of many more scientists into bio-alchemy. Kennedy and Lehninger wrote: “…it has been a general finding that the more highly organized enzyme systems of animal tissue responsible for oxidation of metabolites by molecular oxygen.” [3]. Thus, Lehninger published ‘the general finding of highly organized enzyme system’, but in his textbook Biochemistry, the diacid is burned as well used as building block [8]. Citrmetral opposite is presented. The advantage of disorganization is propagated and stressed in the box “Entropy” [12]. The propagated mathematics/understanding of Lehninger’s citrate cycle, based on changes in concentration, allowed the mis-incorporation of reversibly acting enzymes. In 1951, D. E. Green summarized “…intermediates do not accumulate during the normal activity of the cyclophorase system… (Citric Acid Cycle)” [17]. In other words, 70 years ago, it was known that the amount of carboxylic acid cycle 1.1 illustrates the molecular mechanism fs in the Citric Acid Cycle complex is constant and that a mathematics based on equilibration reaction and changes in concentration is not fit for biological processes, such as the Citric Acid Cycle. On the basis of the PTC hypothesis, the redox-mechanism “metabolic traffic jam” was developed [18]. The metabolic traffic jam ‘pushes’ carboxylic acids out of the complex when a new carboxylic acid is actively transferred into the complex. By doing so, intermediates do not accumulate.
In order to r: synthesis is tegain stoichiometry in the well-established chemical formulae of glycolysis, it was postulated that 3-phosphoglyce ric acid is the product of degradation aPGK. 70 years ago, the stoichiometry to metabolize glucose to two molecules lacH was known. Even Kennedy and Lehninger wrote: “Fluoride was added to inhibit enolase and thereby the tra end-point measured manometrically indicated the formation of 3-phosphoglyceric acid, which causes CO2 liberations fer orom bicarbonate”. Yet, in Lehninger’s textbook Biochemistry the product of the 4thphosphoglycerate kinase (PGK) catalaw of theysed reaction was set 3-phosphoglycerate, a deliberate change from science to alchemy.
Integrating the PTC hypothesis in actual bioscience
Maps of metabolic pathways are remodynamics to biologic process [9]. Textbooks, such as Ainiscent of a railway map. Every molecule is connected to metabolic pathways. Point to one molecule on the map, and we know how the molecule is degraded and synthesized. Such maps give the impression that metabolism is well understood. For example, the effects of insulin on an organism and the signalling pathways of insulin have been investigated for a century; these are well understood. Insulin accelerates the processing of glucose to glucose-1 phosphate and lacH to store excess energy as glycogen and lipids, respectively.
Metabolic Lmaps providehningers Biochemistry, propagate the destination and insulin is the ticket for this route. The PTC hypothesis introduced metabolic switches (enzyme complexes) setting the route and the energy (H+), driving maxterimal enal in one direction.
The PTC hypothesis defined nascent acids as energy carrieropy and thereby exclude ordered structures, such ass driving one metabolic pathway. Enzyme complexes are metabolic switches. The switch-stand GAPDH•LDH-m drives the material in one metabolic pathway, the switch-stand GAPDH•glycerol-3 phosphate dehydrogenase in another metabolic pathway. The same is true for the plasma membranes-located PGK and cytosolic-located PGK.
Malfunctioning switch-stands are linked to obesity, henzyme-complexes,art failure, major depression, chronic inflammation and many more conditions. Genetic variation and/or environmental predisposition are linked to the inaccurate building of the metabolons, organelles,ic flow of energy and material between cells, brief . Briefly, the PTC hypothesis provides mechanisms, mathematics and law of nature for biologic systemal processes.
- Meyerhof O. RECENT INVESTIGATIONS ON THE AEROBIC AND AN-AEROBIC METABOLISM OF CARBOHYDRATES. J Gen Physiol. 1927;8: 531–542. doi:10.1085/jgp.8.6.531
- The Nobel Prize in Physiology or Medicine 1953. In: NobelPrize.org [Internet]. [cited 11 Nov 2020]. Available: https://www.nobelprize.org/prizes/medicine/1953/krebs/lecture/
- Kennedy EP. and Lehninger AL. Oxidation of fatty acids and tricarboxylic acid intermediates by isolated rat mitochondria. JBC 1949 Available: https://www.jbc.org/content/179/2/957.full.pdf
- Roosterman D, Meyerhof W, Cottrell GS. Proton Transport Chains in Glucose Metabolism: Mind the Proton. Front Neurosci. 2018;12. doi:10.3389/fnins.2018.00404
- Roosterman D, Cottrell GS. The two-cell model of glucose metabolism: a hypothesis of schizophrenia. Mol Psychiatry. 2021. doi:10.1038/s41380-020-00980-4
- Roosterman D, Cottrell GS. Rethinking the Citric Acid Cycle: Connecting Pyruvate Carboxylase and Citrate Synthase to the Flow of Energy and Material. Int J Mol Sci. 2021;22. doi:10.3390/ijms22020604
- Roosterman D, Cottrell GS. Astrocytes and neurons communicate via a monocarboxylic acid shuttle. AIMS Neurosci. 2020;7: 94–106. doi:10.3934/Neuroscience.2020007
- The Nobel Prize in Physiology or Medicine 1922. In: NobelPrize.org [Internet]. [cited 25 Dec 2020]. Available: https://www.nobelprize.org/prizes/medicine/1922/summary/
- Glansdorff P, Prigogine I. Thermodynamic Theory of Structure, Stability and Fluctuations. London, New York: John Wiley & Sons Ltd; 1971.
- The Nobel Prize in Physiology or Medicine 1922. In: NobelPrize.org [Internet]. [cited 25 Dec 2020]. Available: https://www.nobelprize.org/prizes/medicine/1922/summary/
- The Nobel Prize in Physiology or Medicine 1953. In: NobelPrize.org [Internet]. [cited 11 Nov 2020]. Available: https://www.nobelprize.org/prizes/medicine/1953/krebs/lecture/
- Kennedy EP. and Lehninger AL. Oxidation of fatty acids and tricarboxylic acid cycle intermediates by isolated rat liver mitochondria. JBC. 1949
- Roosterman D, Meyerhof W, Cottrell GS. Proton Transport Chains in Glucose Metabolism: Mind the Proton. Front Neurosci. 2018;12. doi:10.3389/fnins.2018.00404
- Reed LJ, Hackert ML. Structure-function relationships in dihydrolipoamide acyltransferases. J Biol Chem. 1990;265: 8971–8974.
- Hashimoto T, Hussien R, Brooks GA. Colocalization of MCT1, CD147, and LDH in mitochondrial inner membrane of L6 muscle cells: evidence of a mitochondrial lactate oxidation complex. Am J Physiol Endocrinol Metab. 2006;290: E1237-1244. doi:10.1152/ajpendo.00594.2005
- de Bruijne AW, Vreeburg H, van Steveninck J. Alternative-substrate inhibition of L-lactate transport via the monocarboxylate-specific carrier system in human erythrocytes. Biochim Biophys Acta. 1985;812: 841–844. doi:10.1016/0005-2736(85)90280-9
- Das ML, Koike M, Reed LJ. On the role of thiamine pyrophosphate in oxidative decarboxylation of alpha-keto acids. Proc Natl Acad Sci USA. 1961;47: 753–759. doi:10.1073/pnas.47.6.753
- Becker HM, Klier M, Schüler C, McKenna R, Deitmer JW. Intramolecular proton shuttle supports not only catalytic but also noncatalytic function of carbonic anhydrase II. Proc Natl Acad Sci USA. 2011;108: 3071–3076. doi:10.1073/pnas.1014293108
- Dimmer KS, Friedrich B, Lang F, Deitmer JW, Bröer S. The low-affinity monocarboxylate transporter MCT4 is adapted to the export of lactate in highly glycolytic cells. Biochem J. 2000;350 Pt 1: 219–227.
- Svedružić ŽM, Odorčić I, Chang CH, Svedružić D. Substrate Channeling via a Transient Protein-Protein Complex: The case of D-Glyceraldehyde-3-Phosphate Dehydrogenase and L-Lactate Dehydrogenase. bioRxiv. 2020; 2020.01.22.916023. doi:10.1101/2020.01.22.916023
- Lehninger AL. Biochemistry: The Molecular Basis of Cell Structure and Function. 2nd Edition. New York: Worth Pub; 1978.
- Glansdorff P, Prigogine I. Thermodynamic Theory of Structure, Stability and Fluctuations. London, New York: John Wiley & Sons Ltd; 1971.
- Jørgensen SE. Tentative Fourth Law of Thermodynamics, Applied to Description of Ecosystem Development. Annals of the New York Academy of Sciences. 1999;879: 320–343. doi:10.1111/j.1749-6632.1999.tb10438.x
- Roosterman D, Cottrell GS. The two-cell model of glucose metabolism: a hypothesis of schizophrenia. Mol Psychiatry. 2021. doi:10.1038/s41380-020-00980-4
- Roosterman D, Cottrell GS. Astrocytes and neurons communicate via a monocarboxylic acid shuttle. AIMS Neurosci. 2020;7: 94–106. doi:10.3934/Neuroscience.2020007
- Green DE. The Cyclophorase Complex of Enzymes. Biological Reviews. 1951;26: 410–453. doi:https://doi.org/10.1111/j.1469-185X.1951.tb01205.x
- Roosterman D, Cottrell GS. Rethinking the Citric Acid Cycle: Connecting Pyruvate Carboxylase and Citrate Synthase to the Flow of Energy and Material. Int J Mol Sci. 2021;22. doi:10.3390/ijms22020604