Corynebacterium species are frequent constituents of the normal bacterial flora of the conjunctival sac and are common as well on human skin and mucous membranes and in the intestines.
- Corynebacterium
- infectious keratitis
- conjunctivitis
- resistant bacteria
- fluoroquinolone
- C. macginleyi
1. Introduction
Corynebacterium species are frequent constituents of the normal bacterial flora of the conjunctival sac and are common as well on human skin and mucous membranes and in the intestines [1][2][1,2]. One important species, Corynebacterium diphtheri, is the pathogenic bacterium that causes diphtheria, an upper respiratory infection generally characterized by sore throat and high temperature. In severe diphtheria cases, respiratory obstruction due to the inflammation of the tonsils, oropharynx, and pharynx can lead to death as the worst-case scenario. However, in general, Corynebacterium species seem to have low pathogenicity; therefore, the isolation of Corynebacterium species from infected tissue has been considered to arise as a result of mishandling or contamination [3][4][3,4].
Corynebacterium species can be found commonly on the ocular surface, in conjunction with other indigenous bacteria, such as Staphylococcus epidermidis and Propionibacterium acnes [1][5][1,5]. These various commensal bacteria aid in preventing ocular surface from invasion by foreign organisms [6]. However, in immunocompromised patients, many recent reports have demonstrated that Corynebacterium species can be potentially pathogenic when present on the ocular surface [7][8][9][10][7,8,9,10], as this infection has been associated with cases of endocarditis of the aortic and mitral valves [11], granulomatous mastitis [12], and pelvic osteomyelitis [13]. Many reports have been based on the case series [7][14][15][16][17][18][19][20][21][7,14,15,16,17,18,19,20,21]. Therefore, the frequency of occurrence of various Corynebacterium species on the ocular surface compared to other organs is not well characterized, and the mechanism by which Corynebacterium species function as pathogenic organisms is also unclear. Moreover, the antimicrobial susceptibility pattern in Corynebacterium species, as with other bacteria like Staphylococcus aureus, has been changing in recent years [22]. Consequently, a full understanding of the pathogenicity of Corynebacterium species awaits a systematic review of the various ocular infection cases possibly caused by Corynebacterium species.
2. Corynebacterium Species
The genus Corynebacterium consists of 137 species, and 10 of these have been isolated from corneal infections (Table 1). Corynebacterium species are commonly found in the conjunctiva of healthy adults and have been recognized as non-pathogenic commensal bacteria on the ocular surface [7][8][7,8]. One study of 990 patients prior to cataract surgery revealed positive culture results for Corynebacterium species in the conjunctival sac in 46.4% (460/990), which was much higher than the results for methicillin-sensitive coagulase-negative staphylococci (MS-CNS), at 22.5% (223/990), or methicillin-sensitive Staphylococcus aureus (MSSA), at 4.4% (44/990) [5].
Table 1. Representative cases with ocular surface infection caused by Corynebacterium species.
| Authors (Ref. Number) |
Year | Country | Age | Disease | Type of Strains | Lipophilic or Non-Lipophilic | Past History | Antibiotic Susceptibilities of Corynebacterium Species (Minimum Inhibitory Concentration; (μg/mL)) |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCs | EM | CEPs | LVFX | CPFX | GM/TOB | VCM | CP | |||||||||||||||||||||
| Badenoch PR et al. [14] | 2016 | Australia | 94 | Suture-related keratitis | C. propinquum | Non-lipophilic | Diabetes, Corneal transplantation | 0.02 | >256 (CLDM) | - | - | 0.38 | - | 0.5 | - | |||||||||||||
| Duignan ES et al. [15] | 2016 | Ireland | 52 | Keratitis | C. pseudodiphtheriticum | Non-lipophilic | Corneal inlay | - | - | - | S (MFLX) | - | - | - | S | |||||||||||||
| Todokoro D et al. [16] | 2015 | Japan | 44 | Contact-related keratitis | C. propinquum | Non-lipophilic | Contact lens wear, Proliferative diabetic retinopathy | - | >256 | 0.125 | 1 | 2 | 0.5 | 1 | - | |||||||||||||
| Ruoff KL et al. [17] | 2010 | USA | 84 | Keratitis | C. macginleyi | Lipophilic | Fuchs’ endothelial dystrophy | 0.016 | - | - | - | 0.032 | 0.064 | 0.5 | - | |||||||||||||
| Alsuwaidi AR et al. [18] | 2010 | Canada | 54 | Conjunctivitis | C. macginleyi | Lipophilic | Health care worker | S | R | S | S | S | S | S | S | |||||||||||||
| Inata K et al. [23] | Inata K et al. [27] | 2009 | Japan | 23 | Contact-related keratitis | C. mastidis | Lipophilic | Contact lens wear | - | <0.016 | 0.125 | 0.064 | 0.125 | <0.064 | 0.5 | 4 | ||||||||||||
| Eguchi et al. [8] | 2008 | Japan | 58 | Conjunctivitis | C. macginleyi | Lipophilic | - | - | - | - | >32 | >32 | - | - | - | |||||||||||||
| Eguchi et al. [8] | 2008 | Japan | 72 | Conjunctivitis | C. macginleyi | Lipophilic | - | - | - | - | >32 | >32 | - | - | - | |||||||||||||
| Eguchi et al. [8] | 2008 | Japan | 58 | Conjunctivitis | C. macginleyi | Lipophilic | - | - | - | - | >32 | >32 | - | - | - | |||||||||||||
| Eguchi et al. [8] | 2008 | Japan | 78 | Conjunctivitis | C. macginleyi | Lipophilic | - | - | - | - | >32 | >32 | - | - | - | |||||||||||||
| Suzuki T et al. [19] | 2007 | Japan | 74 | Suture-related keratitis | C. macginleyi | Lipophilic | Corneal transplantation | 16 | 2 | 0.5 | >128 | 128 | <0.13 | 0.5 | - | |||||||||||||
| Suzuki T et al. [19] | 2007 | Japan | 49 | Suture-related keratitis | C. macginleyi | Lipophilic | Corneal transplantation | 16 | <0.13 | 0.25 | 64 | 8 | <0.13 | 0.5 | - | |||||||||||||
| Giammanco G. M et al. [20] | 2002 | Italy | 65 | Corneal ulcers | C. macginleyi | Lipophilic | Trauma | S | S | S | - | S (ENX) | S | S | S | |||||||||||||
| Li A et al. [21] | 2000 | China | 86 | Keratitis | C. pseudodiphtheriticum | Non-lipophilic | Pneumonia, Trichiasis, Lagophthalmos | R | - | R * | - | - | - | S | R | |||||||||||||
| Heidemann DG et al. [24] | Heidemann DG et al. [25] | 1991 | USA | 80 | Keratitis | C. striatum | Non-lipophilic | Proliferative diabetic retinopathy | S | S | S * | - | - | - | S | - | ||||||||||||
| Rubinfeld RS et al. [25] | Rubinfeld RS et al. [26] | 1989 | USA | 81 | Keratitis | C. striatum | Non-lipophilic | Contact lens wear, Aphakia | S | S | S * | - | - | - | S | - | ||||||||||||
| Rubinfeld RS et al. [25] | Rubinfeld RS et al. [26] | 1989 | USA | 11 | Suture related keratitis | C. xerosis | Non-lipophilic | Post corneal laceration | - | S | S * | - | - | S | S | S | ||||||||||||
| Funke et al. [7] | 1998 | Switzerland | 47 | a | Corneal ulcer ( | n | = 3), Conjunctivitis ( | n | = 12) | C. macginleyi | Lipophilic | Contact lens wear, Eyelid closure problems | <0.01–0.125 | b | <0.03–>64 | b | 0.5–16 | b | 0.125–1 (Ofloxacin) | b | 0.06–0.125 | b | <0.06–0.5 | b | 0.5–1 | b | 2–4 | b |
S: Sensitive, R: Resistant, *: First-generation cephalosporins, M: Male, F: Female, a: Average age, b: Range of antibiotic susceptibility in 15 patients, PCs: penicillins, EM: Erythromycin, CEPs: Cepharosporins, LVFX: Levofloxacin, CPFX: Ciprofloxacin, GM: Gentamicin, TOB: Tobramycin, VCM: Vancomycin, CP: Chloramphenicol, MFLX: Moxifloxacin, ENX: Enoxacin, CLDM: Clindamycin.
Hoshi and associates evaluated 183 strains of Corynebacterium species in patients during their preoperative examinations prior to cataract surgery. Positive culture results revealed that C. macginleyi was the predominant strain (84%) in the conjunctiva, followed by C. accolens at 7%, C. propinquum at 3%, C. amycolatum at 2%, C. jeikeium at 2%, and C. mastitidis at 2% [23]. The presence of Corynebacterium species occurs in a tissue-specific manner; for instance, C. macginleyi is a dominant strain in the conjunctiva and was first described by Riegel and associates as a strain isolated from eye samples [24]. By contrast, C. accolens and C. propinquum are two major species in the nasal cavity, at 44% and 31%, respectively, whereas C. macginleyi accounts for only 3% of the isolates from nasal samples [23].
Corynebacterium species is thought to be a possible pathogen responsible for keratitis in some cases, such as biofilm formation in immunosuppressed persons. In fact, cases of Corynebacterium-associated ocular infection, including conjunctivitis, bacteria keratitis, glaucoma bleb-related infections, and endophthalmitis, have been reported [7][8][14][15][16][17][18][19][20][21][24][25][23][26][27][28][29][7,8,14,15,16,17,18,19,20,21,25,26,27,28,29,30,31], have led to suspicion of involvement of Corynebacterium species in immunocompromised cases. Many case reports have implicated C. macginleyi as a main strain that causes conjunctivitis and bacteria keratitis [7][8][17][18][19][20][26][30][7,8,17,18,19,20,28,32].
In this review, we have listed the cases of ocular surface infection, including keratitis, corneal ulcer, and conjunctivitis, caused by Corynebacterium species (Table 1). The identified species were C. macginleyi (n = 24), C. propinquum (n = 2), C. pseudodiphtheriticum (n = 2), C. striatum (n = 2), C. xerosis (n = 1), and C. mastitidis (n = 1) [7][8][14][15][16][17][18][19][20][21][24][25][23][7,8,14,15,16,17,18,19,20,21,25,26,27]. Most of the infected patients had risk factors, such as diabetes or long-term use of steroid treatments after corneal transplantation [14][16][19][24][14,16,19,25] that led to poor immunity, or they had experienced corneal epithelial damage due to trauma [20], contact lens wear [7][16][25][23][7,16,26,27], lagophthalmos, or trichiasis [21]. Interestingly, mild infectious cases, like conjunctivitis, were more often observed in cases of C. macginleyi infection, whereas other Corynebacterium strains seem to cause more severe infectious diseases [14][16][21][14,16,21]. In fact, many severe keratitis cases reported from India were caused by strains identified as C. propinquum (n = 3), C. pseudodiphtheriticum (n = 1), C. striatum (n = 1), and C. bovis (n = 1) [9], suggesting that the causative agents could be either indigenous bacteria or foreign strains.
2.1. C. macginleyi
Riegel and associates reported lipophilic coryneform bacteria from eye specimens that they named C. macginleyi [31][24]. The presence of this species in the normal conjunctiva is not surprising because the meibomian glands produce meibum, an oily substance [31][24]. In fact, C. macginleyi is the most commonly isolated strain in the conjunctiva, and it is also recognized as the most common causative agent of opportunistic ocular infections.
The rDNA sequence of C. macginleyi shows a 98.7% similarity to that of C. accolens. Eguchi and associates showed that C. macginleyi was highly resistant to fluoroquinolones, with 12 of 16 tested isolates showing resistance. They attributed this resistance to overuse of fluoroquinolones eye drops in the ophthalmology field. C. macginleyi is an opportunistic pathogen that causes several ocular infections, including conjunctivitis [7][8][18][7,8,18], corneal keratitis [17][19][17,19], glaucoma bleb-related infections [28][29][30,31], and endophthalmitis [10][27][10,29]. C. macginleyi can also cause non-ocular infections, such as bladder catheter infections [32][33], intravenous catheter infections [33][34][34,35], and septicemia [35][36].
2.2. C. accolens
C. accolens (previously CDC group G-1) was first described from human clinical specimens, such as wound drainage, endocervix samples, sputum, and throat swab specimens collected over a 30-year period [3][36][3,37]. Hoshi and associates revealed that C. accolens was the second most frequent bacterial species, accounting for 7% of the bacteria in the conjunctiva and 44% in the nasal cavity in the healthy volunteers [37][23]. One case with bacterial conjunctivitis had C. accolens identified in the conjunctival sac [8]. However, evidence confirming the Corynebacterium isolates as the causative agents of the infection was not provided.
2.3. C. propinquum
C. propinquum was proposed as the CDC coryneform ANF-3 bacterium by Riegel and associates [38]. Two cases of keratitis caused by C. propinquum have been reported. One case was a 94-year-old woman who had undergone corneal transplantation for Fuchs corneal dystrophy, and she had used long-term 0.1% fluorometholone eye drops as prophylaxis against graft rejection. Corneal infection had occurred at the loosened suture region of the graft. After medication with 5% cefazolin eye drops and 1% gentamicin eye drop hourly and 0.1% fluorometholone eye drops twice daily, her visual acuity improved to 20/160 [14]. Another case was a 44-year-old woman with a persistent corneal epithelial defect. She had a past history of type 1 diabetes, proliferative diabetic retinopathy, and hemodialysis due to diabetic nephropathy. The ocular infection was attributed to a persistent corneal epithelial defect due to neurotrophic keratopathy, possibly caused by poor diabetes control. The patient was treated with gatifloxacin and cefmenoxime eye drops six times daily and complete eye closure with an eye patch to facilitate reepithelialization. Her final visual acuity recovered to 0.02 (20/1000) [16].
C. propinquum is typically commensal on the human nasopharynx and skin [37][39][23,39]. C. propinquum has been implicated in various opportunistic infections, such as pulmonary infection [40] and infective endocarditis [41]. The majority of C. propinquum strains are constitutively resistant to macrolide drugs [37][42][23,42], and both of the above cases were resistant to erythromycin [37][23].
2.4. C. amycolatum
C. amycolatum is a non-lipophilic and fermentative Corynebacterium species, first described by Collins and associates from swabs of the skin of healthy people [43]. One case report has appeared of orbital implant infection after eye evisceration caused by C. amycolatum [44], but no reports of conjunctivitis or keratitis have been published. C. amycolatum is a normal inhabitant of skin and mucous membranes and promotes the epidermal growth factor receptor-dependent induction of the antimicrobial protein RNase7. The relationship between C. amycolatum and RNase7 may control the growth of Corynebacterium species on human skin [45].
2.5. C. jeikeium
The species C. jeikeium was isolated from bacterial endocarditis following cardiac surgery [46]. C. jeikeium is part of the normal flora of the skin, especially in inpatients. Many studies have reported multi-resistance to antibiotics in C. jeikeium strains [47][48][49][47,48,49].
2.6. C. mastitidis, C. lowii, and C. oculi
C. mastitidis was first found in sheep with mastitis [50] and this species can stably colonize the ocular mucosa, where it provides a related beneficial local immunity [51]. Bernard and associates analyzed C. mastitidis recognized by Eguchi in Japan and Vandamm in Belgium and Switzerland [8][52][8,52], and described C. lowii and C. oculi as two new species, separate from C. mastitidis. C. mastitidis was detected in contact-related keratitis in Japan; however, unlike C. macginleyi, C. mastitidis was very sensitive to both levofloxacin and ciprofloxacin [23][27].
3. Laboratory Examinations
3.1. The Ocular Manifestations of Corynebacterium Species
Corynebacterium-associated conjunctivitis is characterized by variable symptoms, including hyperemia, foreign body sensation, discharge, and a burning sensation [8][18][26][30][8,18,28,32]. Bacterial keratitis caused by Corynebacterium species shows clinical features by slit lamp microscopy that range from mild cases with a small infiltration on the surface of cornea to severe cases with corneal ulcerations with round to oval shapes at the center of cornea, but the ocular infections show no distinctive findings [9][17][19][24][53][54][9,17,19,25,53,54]. This infection is more frequently found in patients undergoing immunosuppressive therapy after corneal transplantation. As shown in Table 1, age differences do not seem to affect the ocular infection by Corynebacterium species. Trauma, contact lens wear, and corneal damage due to trichiasis and severe dry eye can also exacerbate the ocular infection [7][15][20][21][25][23][7,15,20,21,26,27]. However, Corynebacterium species isolated as part of the normal flora in the conjunctival sac showed a significant association with age, male sex, and glaucoma eye drop use in a multivariate analysis [5], suggesting that clinical information would be helpful for an accurate diagnosis.
Figure 1 shows the findings for an 89-year-old man who had undergone corneal transplantation for lattice corneal dystrophy eight years previously. He had applied 0.1% fluorometholone eye drops twice daily for the prevention of allograft rejection, accompanied by moxifloxacin eye drops for post microbial keratitis as prophylaxis. At his regular visit, corneal infiltration was found at the suture site. A microbial examination identified Corynebacterium species that were resistant to levofloxacin, erythromycin, cefmenoxime, and fosfomycin and were susceptible to arbekacin, vancomycin, and chloramphenicol. The patient was treated with 0.3% chloramphenicol eye drops, 1% vancomycin ointment, and 1% cefmenoxime eye drops, and the keratitis was resolved.
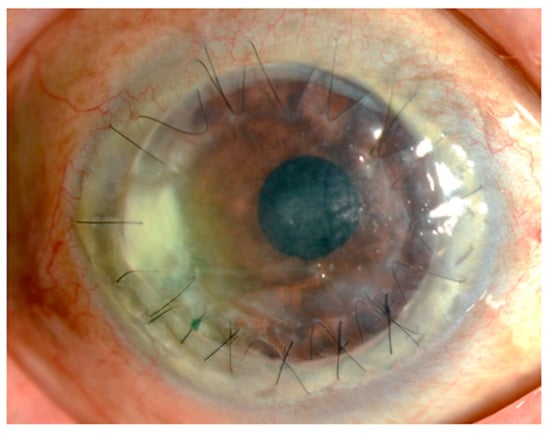
Figure 1. Slit lamp photograph. An 89-year-old man who underwent a penetrating keratoplasty at his age of 81. The corneal infiltration was observed around the graft suture, accompanied with moderate hyperemia.
3.2. Microscopy Examinations
Microscopy examinations of the discharge/fluid from the infected conjunctival sac and of corneal scrapings are helpful for identifying the causative bacteria. Corynebacterium species are gram-positive, rod-shaped, non-branching, non-motile, catalase positive, and oxidase negative bacteria. They grow in aerobic conditions, and Corynebacterium species are widely present in nature, in water, soil, and plants. The range in size from 0.3–0.8 μm in diameter and 1–8 μm in length. They look like the letters “I, N, T, V, W, or Y” at 1000× magnification, and show the apical growth typical of a bacillus, often exhibiting a club-shaped morphology at one or both ends. Corynebacterium species are commonly isolated as indigenous bacteria from the normal ocular flora as well as the mucosa and skin. However, as shown in Figure 2, Corynebacterium species are phagocytosed by polymorphonuclear leucocyte, indicating that they can have a potential impact on infection.
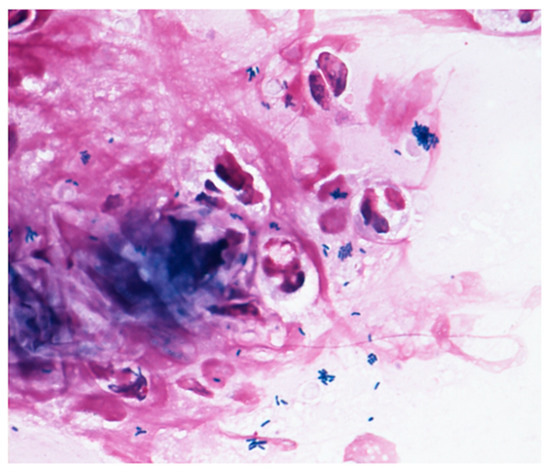
Figure 2. Gram staining. The Gram stain procedure revealed that gram-positive rod-shaped bacteria were phagocytosed by polymorphonuclear leucocyte.
3.3. Culture Tests
Most Corynebacterium species can be isolated from a 5% sheep blood agar, and they often form staphylococcal-like colonies. The growth of lipophilic Corynebacterium species is enhanced by adding 0.1% Tween 80 to the medium, while blood agar under aerobic conditions helps the growth of non-lipophilic Corynebacterium species. C. macginleyi is a lipophilic Corynebacterium and requires lipid for growth. Clinical laboratories typically report these organisms as “Corynebacterium spp.” based on visualization and catalase reactions only, which could lead to misdiagnosis. When other commensal bacteria from the respiratory system material or urinary system material are present, identification of Corynebacterium species may not always be necessary, as they are unlikely to be the causative pathogens of an infection. Therefore, Corynebacterium should be detected in clinical samples from normally sterile sites. However, the normal bacterial flora on the ocular surface includes Corynebacterium species [1], suggesting that the identification of Corynebacterium species should be performed with clinical findings in cases when immunity is severely affected, as those patients could develop infections caused by Corynebacterium species.
3.4. Antimicrobial Susceptibility Testing
Antimicrobial susceptibility testing is a method for determining possible drug resistance and for assuring the susceptibility to the drugs of choice for a particular bacterial species. Antimicrobial susceptibility testing should be performed for cases highly suspicious for Corynebacterium being a causative pathogen based on smear speculation or isolates from sterile materials. The minimal inhibitory concentrations (MIC values) of a drug for lipophilic or non-lipophilic Corynebacterium are typically determined using 5% lysed horse blood added to agar and adjusted with Ca and Mg with microfluid dilution. The bacteria are incubated for 24–48 h at 35 °C, with 48 h needed for lipophilic Corynebacterium species. Growth on control agar is compared to growth on the drug-containing agar to determine susceptibility or resistance. The disk diffusion method is a standardized technique for testing rapidly growing pathogens. However, many reports suggest some limitations to the use of the disc diffusion method [55][56][55,56], as some cases do not accurately display a visual resistance reaction.
