In the 21st century and especially during a pandemic, the diagnosis and treatment of depression is an essential part of the daily practice of many family doctors. It mainly affects patients in the age category 15–44 years, regardless of gender. Anxiety disorders are often diagnosed in children and adolescents. Social phobias can account for up to 13% of these diagnoses. Social anxiety manifests itself in fear of negative social assessment and humiliation, which disrupts the quality of social functioning. Treatment of the above-mentioned disorders is based on psychotherapy and phar-macotherapy. Serious side effects or mortality from antidepressant drug overdose are currently rare. Recent studies indicate that paroxetine (ATC code: N06AB), belonging to the selective sero-tonin reuptake inhibitors, has promising therapeutic effects and is used off-label in children and adolescents.
- paroxetine
- CYP
- MAT
- GRK2
- EBOV
Note: The following contents are extract from your paper. The entry will be online only after author check and submit it.
1. Introduction
Anxiety is an emotional state characterized by feelings of unreasonable fears, feelings of danger, and may have different severity and duration. Once anxiety exceeds personal adaptive abilities, its intensity and duration may be disproportional in relation to the stimulus that has triggered it. This sense of fear is modulated by some regions of the brain such as the amygdala, hippocampus and prefrontal cortex. Incorrect adjustments in the tuning of specific circuit components, including deficiencies in the dampening of amygdala stress responses by prefrontal regions, are involved in alterations in fear response. Clinical anxiety often causes intense need to escape, which may result in immediate relief from symptoms. Such avoidance is so reinforcing that it can quickly become a habit that creates increasingly impaired functioning. Fear is associated with the occurrence of somatization symptoms (rapid heart rate, sweating, tremor, dyspnea, fast breathing) due to vegetative imbalance leading to significant decline in daily functioning [1].
Anxiety disorders (ADs, ICD-10-CM code: F40-F48, [2]) are reported to be the most common mental disorders worldwide. In the USA, they affect 18 percent of the general population, which is more than twice as much as mood disorders (including recurrent depression and bipolar disorder) and twenty-fold more than schizophrenia [3]. The prevalence of ADs in children and adolescents is estimated to be in range from 6 to 20%, placing these diseases among the most common mental disorder/illnesses in developed countries. According to the American Society of Anxiety and Depression (ADAA) estimates, only a third of patients suffering from AD receive adequate help and treatment despite the fact that ADs are highly treatable. In children, before the age of 12, separation anxiety is the most common, and its occurrence decreases with age [3,4]. On the contrary, the most common AD among adolescents is social anxiety disorder (SAD, ICD-10-CM code: F40.1, F40.10, F40.11 [5]) (0.3–13.1%), the spread of which increases with age. The peak incidence occurs at adolescence and early adulthood. Throughout the lifespan, females are more likely to suffer from AD (9.5%) than males (4.9%) [4].
SAD, also called social phobia, is the second most common AD and is characterized by fear of public assessment and humiliation. The disorder leads to significant disturbances in social functioning of a patient [6]. Despite the availability of effective treatments, fewer than 5% of people suffering from SAD seek professional help during the first year after the initial onset [3,7,8].
Treatment of the above-mentioned disorders is based on psychotherapy and pharmacotherapy. In case of child and adolescent patients, only the former is approved as the first line. However, recently, it often happens that pharmacotherapy is introduced using some unapproved substances. Prescription outside the indications occurs when a child receives a drug that has not been approved by the appropriate agency (e.g., FDA or EMA) for a given diagnosis or child patient age. This so-called off-label prescribing frequently occurs despite the lack of information on medication safety, efficacy and proper use in children (e.g., dosing and interactions). Furthermore, off-label prescribing has been associated with adverse drug events [9].
Paroxetine (ATC code: N06AB [10]; DrugBank ID: DB00715 [11]) belongs to the SSRI group (selective serotonin reuptake inhibitors,) and according to the literature, it is one of the most common off-label drugs used in daily clinical practice [12]. The plethora of publications devoted to the drug deliver a variety of information concerning different aspects of the drug’s use and modes of action. Nevertheless, there is a lack of papers devoted to explanation of paroxetine’s mechanism of action on specific targets based on X-ray-supported structural studies. Unapproved prescription of medicines to children has been rated to fall in the range between 11 and 79% worldwide. Serious side effects or mortality from antidepressant drugs overdose are currently rare [13]. Paroxetine was estimated to be among the five most commonly used drugs for depression in children [14]. Recent studies indicate that paroxetine has promising therapeutic effects and is used off-label in children and adolescents [15]. In a natural way, these facts clearly indicate the need to discuss the possibility of using paroxetine as indicated for young patients. For this reason, the main issue of this work is the presentation of data related to the description of paroxetine activities tested at the molecular level based on the collected crystallographic data.
The purpose of this review is focused on description of the interaction of paroxetine with several molecular targets from various perspectives. In the paper, the basic chemical and pharmaceutical properties of paroxetine are discussed to provide a proper foundation for further discussion. Later in the text, detailed pharmacodynamic analyses are drawn based on the molecular mechanism of paroxetine’s binding to all available therapeutic targets that have structure confirmed by X-ray studies. Binding of a ligand to a specific target protein requires a specific arrangement of both the ligand and binding site in the protein. The attractive interactions between ligand and target usually have the nature of noncarbonated contacts (hydrogen bonding, Van der Waals interactions and so on). Detailed characterization of the interaction patterns between paroxetine and analyzed proteins was carried out in the course of this study. For each biological activity discussed here, a detailed 2D representation of protein–ligand interactions is presented using LigPlot+ software [16]. A uniform method of visualization was adopted to better represent the interaction for all the therapeutic targets analyzed.
2. Paroxetine—General Information and History
Paroxetine is a drug indicated for the treatment of variety of anxiety disorders, including generalized anxiety disorder (GAD, ICD-10-CM code: F41.1 [17]), obsessive–compulsive disorder (OCD, ICD-10-CM code: F42.3, F42.2, F42.9, F42.8 [18]), major depressive disorder (MDD, ICD-10-CM code: F32, F33 [19]), premenstrual dysphoric disorder (PDD, ICD-10-CM code: F32.81 [20]), post-traumatic stress disorder (PTSD, ICD-10-CM code:F43.1 [21]), panic disorder (PD, ICD-10-CM code: F41.0 [22]), social anxiety disorder (SAD, ICD-10-CM code: F40.1, F40.10, F40.11 [5]) and vasomotor symptoms [23–26]. It is worth emphasizing that in the treatment of PTSD there are only two approved pharmacotherapies based on SSRIs, including Paxil (paroxetine hydrochloride). In all of the above disorders, pharmacotherapy is used in children and adolescents with off-label markings. First-line treatments for depression among children are nonpharmacological approaches [13,27].
Preliminary evidence indicates better therapeutic efficacy of paroxetine in children and adolescents with OCD, social phobia or depression when compared to that in adults [28]. However, there have been reports in the literature indicating the influence of paroxetine therapy in children and adolescents suffering from severe depressive disorders on the increase of suicidal tendencies, as compared to placebo [29–37]. For this reason, no antidepressants, including paroxetine, are contraindicated in children [34,38,39]. Antidepressants are used to treat depression and prevent disease-induced suicide, the possibility that drug-induced suicide can appear as side effect is a serious issue that needs to be thoroughly investigated [27,38–41]. Existing studies on the link between suicide and antidepressants vary with different results and continue to cause a lot of controversy [32–36]. In addition, it is estimated that the risk of negative effects of untreated or undertreated depression is usually higher than the risk of drug-induced suicide [27,41].
Paroxetine is an active substance of drugs known by the trade names Aropax, Paxil, Pexeva, Seroxat, Sereupin and Brisdelle. It was first marketed in the U.S. in 1992 under the proprietary name Paxil [42]. It is administered orally as a solid dose tablet, oral suspension, or controlled-release tablet [43]. In its clinical efficacy paroxetine can be compared with tricyclic antidepressants; however, it is safer and has greater acceptance by the patients [44,45]. According to the information provided by Paxil manufacturer GlaxoSmithKline and approved by the FDA, the effectiveness of this drug in MDD has been proven by six placebo-controlled clinical trials. For panic disorder, three 10–12-week studies indicated paroxetine’s superiority to placebo. Similarly, three 12-week trials for adult outpatients with social anxiety disorder demonstrated better response to paroxetine than to placebo [46–48]. It has also been used in the treatment of diabetic neuropathy, vasovagal syncope and chronic headache [49]. Paroxetine also has proven effective in the treatment of vasomotor symptoms (e.g., hot flashes, night sweats) in women undergoing menopausal transition and in patients receiving antiestrogenic cancer therapy [50]. Paroxetine is also used as a veterinarian medicine. It has been proven useful in the treatment of canine aggression and stereotyped or another OCD behavior. It has also been used in cats from time to time [51].
In pharmacological studies, various tests were conducted to confirm the expected biological activity, e.g., for serotonin transporter (SERT) inhibition or to test a specific mechanism of action as is the case in Ebolavirus (EBOV) studies. There has also been an accidental discovery of unexpected activity towards disorders in the circulatory system. Table 1 summarizes the results of crystallographic paroxetine studies from different perspectives.
Table 1. The list of the crystal structure of target bounded Paroxetine.
|
PDB ID |
Target |
Resolution |
Organism |
Reference |
|
5I6X, 5I6Z, |
SERT |
3.14 Å, 4.53 Å |
Homo sapiens, Mus musculus |
[52] |
|
6AWN |
SERT |
3.62 Å |
Homo sapiens, Mus musculus |
[53] |
|
6VRH |
SERT |
3.30 Å |
Homo sapiens, Mus musculus |
[54] |
|
4JLT |
P450 2B4 |
2.14 Å |
Oryctolagus cuniculus |
[55] |
|
4L9I |
GRK1 |
2.32 Å |
Bos taurus |
[56] |
|
3V5W |
GRK2 |
2.07 Å |
Bos taurus, Homo sapiens |
[57] |
|
4MM4 |
LeuBAT |
2.89 Å |
Aquifex aeolicus VF5 |
[58] |
|
6F6I |
EBOV GP |
2.40 Å |
Ebola virus |
[59] |
Facts related to therapeutic costs cannot be ignored as well. According to GlobalData Projects Drug estimates increase of sales for PTSD in the seven major markets (7MM: the US, France, Germany, Italy, Spain, the UK and Japan) from $211.4 million in 2018 to $1.2 billion in 2028, at a CAGR of 18.7% [60]. The cited data demonstrate the scale of this issue. Nevertheless, this thread is beyond the scope of the present review.
3. Paroxetine—Chemistry
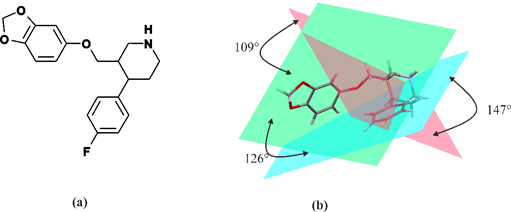
Paroxetine is a phenylpiperidine derivative. It is composed of a secondary amine residing in the piperidine ring, which in turn is connected to benzodioxol and fluorophenyl groups [54] (Figure 1). From a chemical point of view, paroxetine is enantiomerically pure, (−)-(3S,4R)-3-[(2H-1,3-benzodioxol-5-yloxy)methyl]-4-(4-fluorophenyl)piperidine hydrochloride hemihydrate with empirical formula of C19H20FNO3·HCl·½H2O (PubChem CID: 43815 [61]). It is an odorless, off-white powder, having a melting point ranging between 120 and 138 °C. Particularly, paroxetine is a relatively small molecule with molecular weight of 374.8 g/mol (329.4 g/mol as free base). [47]. In addition to hydrochloride, paroxetine mesylate is also available [23]. It can be concluded that nowadays, the structure of paroxetine is well-researched and understood (Figure 1). The compound exists in two crystal forms, i.e., a stable hemihydrate referred to as form I and the anhydrous form called form II (CCDC DN: 125003 [62]) [63,64]. Spectroscopic data are available in the literature and databases: FTIR (SpectraBase Compound ID: 31iWZ0aC88k [65]) NMR ([66]) MS (accession: AU152606 [67]). These data clearly show that each of the three rings present in paroxetine structure is located on a different plane, so the structure of this compound is a highly nonplanar molecule, as is depicted in Figure 1.
Figure 1. Molecular structure of paroxetine: 2D semi-structural scheme (a) and 3D stick representation with indicated planes of rings. The pink plane corresponds to the piperidine ring, green one to the benzodioxol ring and blue one to the fluorophenyl ring (b).
It is a lipophilic base amine with both hydrophobic and hydrophilic moieties (pKa is 9.9 and the partition coefficient of paroxetine (log Po/w = 3.95)) [44]. It is slightly soluble in H2O (5.4 mg/mL), sparingly soluble in Me2Cl2 and EtOH (96%) but entirely soluble in MeOH. The chemical properties of this compound make it easy to modify and preserve drug-like properties [43].