This article explains this product's requirements and reviews the developmental steps and published literature on the use of Suprathel® (Polymedics Innovations GmbH. Denkendorf, Germany).
Successful research and development cooperation between a textile research institute, the German Federal Ministry of Education and Research via the Center for Biomaterials and Organ Substitutes, the University of Tübingen, and the Burn Center of Marienhospital, Stuttgart, Germany, led to the development of a fully synthetic resorbable temporary epidermal skin substitute for the treatment of burns, burn-like syndromes, donor areas, and chronic wounds. This article describes the demands of the product and the steps that were taken to meet these requirements.
The material choice was based on the degradation and full resorption of polylactides to lactic acid and its salts. The structure and morphology of the physical, biological, and degradation properties were selected to increase the angiogenetic abilities, fibroblasts, and extracellular matrix generation. Water vapor permeability and plasticity were adapted for clinical use. The available scientific literature was screened for the use of this product. A clinical application demonstrated pain relief paired with a reduced workload, fast wound healing with a low infection rate, and good cosmetic results.
A better understanding of the product’s degradation process explained the reduction in systemic oxidative stress shown in clinical investigations compared to other dressings, positively affecting wound healing time and reducing the total area requiring skin grafts.
- burn
- donor area
- wounds
- polylactide
- lactormone
- oxidative stress reduction
- analgesia
- stabili-zation
- reduced infection
Note: The following contents are extracted from your paper. The entry will be online only after author check and submit it.
1. Introduction
1. Introduction
Stuttgart is one of Germany’s most innovative areas, as the location of the headquarters and development centers of several multinational automobile companies, including Daimler AG (Mercedes) and Porsche. Alongside a university and a technology university of applied sciences, many research companies are located there.
Stuttgart is one of Germany’s most innovative areas, as the location of the headquarters and development centers of several multinational automobile companies, including Daimler AG (Mercedes) and Porsche. Alongside a university and a technology university of applied sciences, many research companies are located there.
Among these is the German Institutes for Textile and Fiber Research Denkendorf (DITF), the largest textile research center in Europe with more than 300 scientific employees. The company’s objective is to conduct research projects that ultimately lead to developing degradable materials that do not cause any harm the environment. The idea of using such a product as a medical wound dressing was the underlying idea behind Suprathel
Among these is the German Institutes for Textile and Fiber Research Denkendorf (DITF), the largest textile research center in Europe with more than 300 scientific employees. The company’s objective is to conduct research projects that ultimately lead to developing degradable materials that do not cause any harm the environment. The idea of using such a product as a medical wound dressing was the underlying idea behind Suprathel
®
. Developing a degradable synthetic skin substitute is a convincing approach to tackling the shortcomings of burn care and biological skin substitutes.
A joint venture between the Institute of Textile Research and Chemical Engineering Denkendorf and Stuttgart Burn Center at the Clinic for Orthopedics, Trauma Surgery and Sports Traumatology of Marienhospital, Stuttgart, Germany, was formed within the scope of the BMOZ (German Center for Biomaterials and Organ Substitute Stuttgart–Tübingen
)
, supported by the German Federal Ministry of Education and Research, the Federal Country of Baden-Württemberg, and the University of Tübingen to develop a material “combining the advantages of biological and synthetic substitutes.”
This material was needed to advance skin substitution processes to improve burn and wound care. A transparent, permanent wound dressing was to be developed, allowing the proper assessment of the treated wound. It needed to serve as an epithelial replacement until the wound had healed completely, enabling both analgesia and the damaged epithelium's undisturbed regeneration. The durability also had to prevent infection or iatrogenic compromise when changing the dressing. Dressing changes, if necessary, had to be as painless and straightforward as possible.
Later on, a spin-off of the institute was founded (PolyMedics Innovations GmbH) to further investigate said medical dressing and promote its development.
This article explains the requirements of this product and reviews the developmental steps and published literature on the use of Suprathel
®
(Polymedics Innovations GmbH. Denkendorf, Germany)
2. Technical Features in the Development of a Skin Substitute
2.1. The Choice of Materials
It was an obvious choice to use materials from components already available in the healthy human metabolism. Hydrocarbons are common and usually present in a cyclic form. As the material needs some stability over a specific time, which should reduce during degradation and resorption, polymers of lactic acid seemed to be suitable as an essential product. Polymers with a higher molecular weight degrade in a humid environment into monomers of lactic acid, buffered physiologically. It has been demonstrated that externally applied lactic acid at a lower concentration positively influences keratinocyte-derived growth factors such as VEGF (Vascular Endothelial Growth Factor. Lactates, the salts of lactic acid, are known to scavenge superoxide radicals and inhibit lipid peroxidation. When lactate oxidizes to pyruvate, this molecule can scavenge hydrogen peroxide and superoxide radicals as well. Both lactic acid and lactate can enter the cells. Due to its small molecules and lack of polarity, Lactic acid, being small enough to permeate through the lipid membrane, can enter cells via the monocarboxylate transporter (MCT) protein shuttle system. Once inside the cell, lactate can serve as an energy source via the Cori cycle, wherein it is converted into glucose. Lactate does not cause acidosis within a physiological range.
Lactate dehydrogenase can alternatively convert lactate into pyruvate, which is oxidized to acetyl Co-A, producing water, carbon dioxide, and NADH (nicotinamide adenine dinucleotide plus hydrogen) in the mitochondria. It can serve as an energy source and as an energy transport system.
2.1.1. The Chemical Structure
The chemical structure influences the degradation time, potential toxicity, and foreign body reactions in a wound environment. The initial molecular weight and structure and the chirality of polylactides influence the speed of degradation and the degree of crystalline products' formation. Schakenraad showed, in 1991, that the initial hydrolysis of amorphous poly-l-lactide might increase crystallinity and lead to a long degradation time. Therefore, the challenge was to develop a chemical compound without developing crystallinity and a foreign body reaction with a shorter resorption time.
2.1.2. The Material Structure
The material had to provide a certain degree of stability, as it was intended primarily for temporary use in place of human biological skin. This was combined with other human skin qualities such as water vapor permeability and mechanical strength, flexibility, and adherence to the wound bed.
The impact of morphology on the degradation and resorption speed was described by Taylor et al. Different structures, resulting in a different porosity, showed a different impact on angiogenesis. Many materials with different structures and thicknesses, such as foams, fleeces, and hot melt blows, were analyzed. The angiogenic response, structural stability, and degradation had to be balanced not to create hypertrophic granulation tissue, provide sufficient water vapor permeability and stability, and preserve a moist wound environment. A balance of these properties would allow the product to be used for partial-thickness burns, supporting wound healing and the epithelium's regeneration without generating granulation tissue. These considerations finally resulted in the pore size and structure used in Suprathel
®
(Polymedics Innovations GmbH, Denkendorf, Germany).
3. Results of the Development Process
3.1. Material Properties:
This development process resulted in a specific microporous structure for Suprathel
®
. This microporous structure provides an increased moisture vapor permeability at the beginning of the treatment. Induced by fibrine and the outer dressing, the water vapor permeability of Suprathel
®
is reduced over time to that of healthy human skin.
Suprathel
®
consists of DL-lactide (>70%), trimethylene carbonate, and ε-caprolactone. A unique processing technique creates a porous membrane with a nearly symmetrical cross-section, with an interconnected pore structure and varying pore sizes between 2 and 50 μm. The initial porosity of the material is >80%. Due to its plasticity, it adapts to the wound bed at body temperature. It promptly adheres to a fresh wound. The membrane has a thickness of 70–150 μm and an elongation potential between 100 and 250%. Storage at room temperature is possible for three years without the loss of quality. Degradation and loss of stability occur within four weeks in vitro.In vivo, it is stabilized by fibrin, and the separation layer and remnants are removed after wound healing. Examples for the use of Suprathel
®
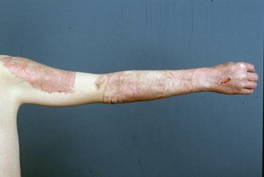
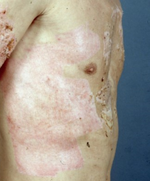
are shown in Figures 1 and 2a,b.
Figure 1.
Shows the first application of Suprathel
®
in 1999 in a donor site (courtesy of Dr. Rapp).
 |
 |
|
(a) |
(b) |
Figure 2.
(
a
)
Shows healed areas after the application of Suprathel on a mesh-grafted area, and (
b
) shows a healed donor area after Suprathel application on day 13 post-op. (courtesy of Dr. Rapp).
3.2. Medical Aims of Suprathel Development
Table 1 gives an overview of the medical key points of the development of Suprathel
®
. The effects are described in detail in the following paragraphs.
Table 1.
Medical and handling key points of the development of Suprathel
®
.
|
· Pain reduction; |
|
· reduction in oxidative stress and the systemic inflammatory response; |
|
· Reduction in the need for skin transplantations and consecutive donor wounds; |
|
· Support for wound healing and more efficient healing; |
|
· Low infection rate; |
|
· Good cosmetic results and scar quality; |
|
· Reduction in workload; |
|
· Economic efficiency. |
