Obstructive sleep apnea (OSA) is a common sleep disorder that affects all age groups and is associated with many co-morbid diseases (especially cardiovascular diseases). Continuous positive airway pressure (CPAP) is the gold standard for treating OSA. However, and with increasing prevalence of CPAP non-adherence, other therapeutic interventions have emerged. Hypoglossal nerve stimulation is a novel modality of treating patients with moderate to severe OSA who are not adherent to CPAP.
- obstructive sleep apnea
- hypoglossal nerve stimulation
- DISE
1. Introduction
Obstructive sleep apnea (OSA) is a prevalent condition affecting approximately three to seven percent of males and two to five percent of females in the adult population[1] [1]. Untreated OSA is associated with multiple comorbidities including hypertension [1], diabetes mellitus [2], coronary artery disease [3], stroke [4], congestive heart failure [5], atrial fibrillation [6] [6], and death [7]. Untreated OSA is also associated with decreased quality of life indicators for patients and excessive daytime sleepiness [8]. Obstructive sleep apnea has a large economic impact when left untreated [9].
Obstructive sleep apnea is characterized by repetitive upper airway collapse during sleep [10]. Upper airway patency is maintained by contraction of the upper airway dilator muscles [10]. There are several upper airway dilator muscles [10]. The most important upper airway dilator muscle is the genioglossus muscle which has phasic activity during inspiration. The genioglossus muscle is innervated by the hypoglossal nerve (cranial nerve XII) [10]. Other dilator muscles such as the tensor palatini stiffen the upper airway by having tonic activity throughout inspiration and expiration [10]. The upper airway dilator muscles are generally effective in maintaining upper airway patency except when limited by anatomy (e.g., retrognathia [11]) or excessive loading from soft or fatty tissue [12]. Ensuring upper airway patency is the underlying mechanism of action of all OSA treatment methods [13].
There are many options for treating obstructive sleep apnea including weight loss, positional therapy (i.e., encouraging side sleeping), mandibular advancement devices, positive airway pressure therapy (PAP), and surgery [13]. As can be expected, weight loss is difficult to achieve and usually takes several years prior to being successful [14]. Positional therapy, effective only in a subset of patients, can be uncomfortable and often difficult for patients to adhere to [15]. Mandibular advancement devices may only be effective in mild and moderate cases of OSA and need the availability of a qualified dentist to fabricate the device [13][16][13,16]. Positive airway pressure therapy is very effective, however not well tolerated by many patients [13][16][13,16]. Finally, conventional surgical options including septoplasty, nasal polypectomy, adenoidectomy, tonsillectomy, uvulopalatopharyngoplasty, uvuloplasty, glossectomy, tongue base reduction, mandibular advancement, genioglossal advancement, hyoid myotomy suspension, maxillomandibular advancement, tracheostomy, and bariatric surgery can be quite invasive and have varying success rates depending on the surgery from 35 to 86% [17]. Upper airway stimulation (UAS) is a more recent surgical option for treating obstructive sleep apnea with a success rate of about 75% at 5 years[18] [18].
2. Implantation Process (Surgical Technique), Pre- and Post-Implant Procedures, and Follow Up
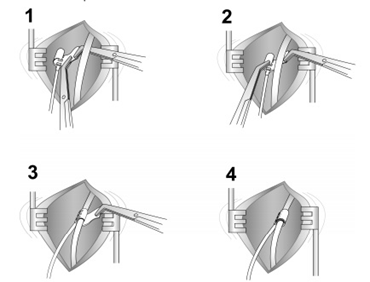
The current FDA-approved Inspire device is placed during what is typically an outpatient procedure requiring general anesthesia that lasts between 90 and 180 min and requires three small skin incisions [19][25]. The first incision is placed typically in the right upper neck midline between the hyoid and mandible. After retracting the submandibular gland posterosuperior, the distal hypoglossal nerve is identified. The cuff is then placed around the nerve and irrigated with sterile saline. Cuff placement is tested using the generator or an external nerve stimulator, or recently, a nerve monitoring-guided selective hypoglossal nerve stimulator [20][26]. The second incision is made in the chest on the same side as the neck incision for the generator pocket where the pulse generator is implanted. The third incision is made on the same side for placement of a pleural respiratory sensor (Figure 9). All components are connected via leads that are subcutaneously tunneled. Electrical testing is performed at the time of surgery to ensure satisfactory tongue movement and adequate respiratory signal waveform before the incisions are closed. A postoperative chest X-ray is obtained for documentation of baseline device position and to rule out negative sequelae such as a pneumothorax. Such complications are rare, as the procedure does not directly involve the airway [21][27].
Figure 9. The neck incision (placing the cuff around the hypoglossal nerve). The steps of placing the cuff around the hypoglossal nerve 1–4.
The role of sleep medicine providers is to identify OSA patients who have problems tolerating traditional CPAP therapy or CPAP failure. If they have an interest in HGNS, they are further screened with a complete health history, sleep comorbidities, and BMI. They undergo overnight diagnostic PSG (Polysomnography; this is discussed in detail in the next section).
If they meet the criteria, then the next step is referral to ENT where evaluation of the upper airway endoscopically is performed utilizing Drug-Induced Sleep Endoscopy (DISE), specifically looking for anteroposterior tongue base and palate collapse. Patients with complete concentric collapse at the level of the velum are poor candidates. Sleep providers also assist with titration of the device for optimal outcomes [22][28]. Following the implantation process, a follow up with ENT in four weeks is usually scheduled to assess for healing and any adverse events. During this time, the generator remains deactivated. Six weeks after implantation, the patient visits with a sleep provider to activate the generator (this process was discussed earlier in this review). Two months later, a full-night lab titration study is conducted to ensure the effectiveness of the Inspire settings (mainly functional threshold) in all sleep stages and in all sleep positions. Then, follow up visits at 3, 6, and 12 months can be scheduled to evaluate the long-term clinical outcome [23][29].
DISE is a preoperative requirement in patient selection for HGNS. The airway responds differently during wakefulness and sleep; during sleep, the upper airway has limited tone and muscle control. DISE mimics the airway during sleep, giving a more accurate picture of the degree of collapse and phenotype of collapsibility. Propofol, midazolam, and dexmedetomidine are the drugs of choice for DISE. The degree of sedation is important; a light sedation that most closely mimics sleep is ideal for accurate assessment of the upper airway in the context of selection of surgical candidates [24][30]. In patients with complete centric collapse (CCC), HGNS is associated with poor outcome [22][28].
3. The Clinical Indications, Contraindications, and Requirements for HGNS Consideration
Upper airway stimulation (UAS) is indicated in patients with moderate and severe OSA (AHI greater more or equal to 15 events per hour and less than or equal to 65 events per hour) who cannot tolerate or failed positive airway pressure (PAP) therapy. PAP failure is defined as persistent elevation in the apnea–hypopnea index (AHI ≥ 15 events per hour), while PAP intolerance is defined as the inability to use PAP therapy continuously (more than or equal to five nights per week for more than or equal to four hours every night) or the unwillingness to use PAP therapy again after quitting in the past. UAS is used in patients older than 22 years of age. However, it is still indicated in patients between 18- and 21-years-old, especially if adenotonsillectomy is contraindicated or they meet the previously noted criteria. Recently, the FDA approved using UAS in patients between 18 and 21 years [25][31].
UAS is contraindicated in patients with central sleep apnea (defined as a central apnea–hypopnea index of more than 25% of the total AHI), and in patients with sleep-related hypoxia or hypoventilation (such as patients with severe obstructive or restrictive pulmonary diseases). One of the factors that has been shown to be significantly correlated with the success of UAS is body mass index (BMI). BMI ≥ 32 kg/m
2 is less likely to be associated with a successful outcome from UAS. The safety of UAS is still to be proven in pregnant patients and should not be attempted. As mentioned earlier in this review, one of the essential steps before considering UAS is the DISE exam. A complete concentric collapse (CCC) pattern of the palate during each apnea or hypopnea predicts a poor therapeutic success with UAS compared to palatal collapse in the anteroposterior axis [25].
is less likely to be associated with a successful outcome from UAS. The safety of UAS is still to be proven in pregnant patients and should not be attempted. As mentioned earlier in this review, one of the essential steps before considering UAS is the DISE exam. A complete concentric collapse (CCC) pattern of the palate during each apnea or hypopnea predicts a poor therapeutic success with UAS compared to palatal collapse in the anteroposterior axis [31].
HGNS (Inspire) should be considered carefully and cautiously in patients who require frequent Magnetic Resonance Imaging (MRI) scanning. Older Inspire models are not eligible for MRI use and the current Inspire is MRI conditional where they are compatible with MRI scanning of the upper/lower extremities, head, and neck only [26][32].