Cartilage calcification impairs the function of knee joints. Vitamin K can prevent cartilage calcification by regulating the carboxylation of matrix proteins, such as matrix gla protein. Prevention of calcium crystal formation also suppresses inflammation cytokines production by joint tissues.
- matrix gla protein
- calcification
- gla-rich protein
- joint
- cartilage
1. Introduction
Calcium crystals in the joint induced proliferation of synoviocytes and release of inflammatory cytokines and matrix metalloproteinases. Mineralization also led to cartilage stiffness and weakened the function of cartilage as a shock-absorbent [1]. Thus, joint calcification could initiate and exacerbate the progression of Osteoarthritis (OA), and presented as a target for the action of vitamin K.
2. Mechanism of Action
Carboxylation of matrix proteins, which is a functional activity of vitamin K, has been investigated as the action of vitamin K on chondrocytes. Chondrocytes from organ donors with OA produced mainly uncarboxylated matrix gla protein (MGP) vesicles with fetuin per se, while chondrocytes from normal donors produced fully carboxylated MGP and fetuin complexes [2]. MGP can inhibit BMP-2 and -4 [3][4], thereby, suppressing calcification of the joint and formation of osteophyte. Alpha-fetuin acts as a carrier of MGP because it binds with fully carboxylated MGP and can be taken up by the chondrocytes through endocytosis [2].
Since OA is a localized inflammatory event of the joint, protein carboxylation activities of vitamin K could prevent inflammation and prevent the progression of OA. Indirect evidence from chondrocytes of rabbit with OA transfected with γ-glutamyl carboxylase generated increased type II collagen, which is indicative of good chondrocyte survival and differentiation, and lower metalloproteinase and type X collagen, which is indicative of chondrocyte apoptosis. Protein and gene expressions of tumor necrosis factor and interleukin-1 beta were also decreased. Gla-rich protein (GRP) is a vitamin K-dependent protein expressed in the soft tissues of humans and involved in pathological calcification [5]. As mentioned previously, cartilage calcification is a pathological feature of OA [1]. Initial assessment by Cavaco et al. [6] showed that treatment with undercarboxylated and carboxylated GRP inhibited inflammation of chondrocytes and synoviocytes stimulated with interleukin-1β, as evidenced by lower cyclooxygenase II, metalloproteinase 13, and prostaglandin E-2 expression [6]. Treatment with undercarboxylated and carboxylated GRP was shown to reduce tumor necrosis factor-alpha and prostaglandin E-2 levels secreted by lipopolysaccharide- and hydroxyapatite-stimulated THP-1 monocytes. THP-1 cells overexpressing GRP also showed lower gene expression of interleukin-1β, nuclear factor kappa-light-chain-enhancer of activated B cells, and tumor necrosis factor-alpha when challenged with lipopolysaccharide or hydroxyapatite [7]. Thus, this study showed that protein carboxylation might not affect the anti-inflammatory activities of GRP. Yet, THP-1 might not reflect the normal functions of primary macrophages and monocytes because they are immortalized.
The mechanism of actions of vitamin K in preventing OA is shown in Figure 1.
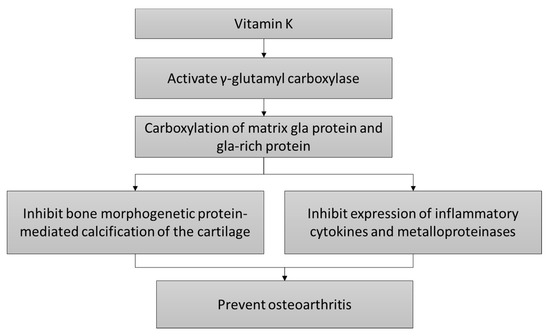
Figure 1. The cartilage protective mechanism of vitamin K.
References
- Hang Korng Ea; Christelle Nguyen; Dominique Bazin; Arnaud Bianchi; Jérôme Guicheux; Pascal Reboul; Michel Daudon; Frédéric Lioté; Articular cartilage calcification in osteoarthritis: Insights into crystal-induced stress. Arthritis & Rheumatism 2010, 63, 10-18, 10.1002/art.27761.
- Reidar Wallin; L.J. Schurgers; R.F. Loeser; Biosynthesis of the vitamin K-dependent matrix Gla protein (MGP) in chondrocytes: a fetuin–MGP protein complex is assembled in vesicles shed from normal but not from osteoarthritic chondrocytes. Osteoarthritis and Cartilage 2010, 18, 1096-1103, 10.1016/j.joca.2010.05.013.
- Yucheng Yao; Amina F. Zebboudj; Esther Shao; Martin Perez; Kristina Boström; Regulation of Bone Morphogenetic Protein-4 by Matrix GLA Protein in Vascular Endothelial Cells Involves Activin-like Kinase Receptor 1. Journal of Biological Chemistry 2006, 281, 33921-33930, 10.1074/jbc.m604239200.
- Amina F. Zebboudj; Minori Imura; Kristina Boström; Matrix GLA Protein, a Regulatory Protein for Bone Morphogenetic Protein-2. Journal of Biological Chemistry 2001, 277, 4388-4394, 10.1074/jbc.m109683200.
- Carla S.B. Viegas; Sofia Cavaco; Pedro Leão Neves; Ana Ferreira; Alexandre João; Matthew K. Williamson; Paul A. Price; M. Leonor Cancela; Dina C. Simes; Gla-Rich Protein Is a Novel Vitamin K-Dependent Protein Present in Serum That Accumulates at Sites of Pathological Calcifications. The American Journal of Pathology 2009, 175, 2288-2298, 10.2353/ajpath.2009.090474.
- Sofia Cavaco; Carla S.B. Viegas; Marta Rafael; Acácio Ramos; Joana Magalhães; Francisco J. Blanco; C. Vermeer; Dina C. Simes; Francisco J Blanco; Gla-rich protein is involved in the cross-talk between calcification and inflammation in osteoarthritis. Cellular and Molecular Life Sciences 2015, 73, 1051-1065, 10.1007/s00018-015-2033-9.
- Carla S.B. Viegas; Rúben M. Costa; Lúcia Santos; Paula A. Videira; Zélia Silva; Nuna Araújo; Anjos Macedo; António Pedro Matos; Cees Vermeer; Dina C. Simes; et al. Gla-rich protein function as an anti-inflammatory agent in monocytes/macrophages: Implications for calcification-related chronic inflammatory diseases. PLOS ONE 2017, 12, e0177829, 10.1371/journal.pone.0177829.
