Trichuriasis is the clinical disease of animals infected with the parasite of the genus Trichuris. This review attempts to present information on Trichuris spp. infestation in neo-tropical rodents that are utilized for meat consumption by humans
- agouti
- lappe
1. Introduction
The neo-tropics is a geographical region located in the western hemisphere between the Tropic of Cancer and the Tropic of Capricorn. Geographical territories present within this zone include the southern parts of North America, all of Central America, the northern parts of South America, and all of the Caribbean[1]. Animals that are present in this region can be categorized into three groups: imported domesticated animals[2], domesticated animals originating from the neo-tropics[3], and non-domesticated neo-tropical animals[4]. For the purpose of this review, neo-tropical rodents that are included belong to the domesticated and non-domesticated groups. Domesticated neo-tropical rodents, such as the guinea pig, are utilized in South America for their meat and are reared in captivity to provide meat protein for rural villages. The guinea pig is able to utilize household waste and provide income and food for these communities[5][6]. Neo-tropical rodents on the verge of domestication are the agouti, lappe, and capybara. These animals have been reared in captivity in South America and the Caribbean for their meat [1]. These animals have been able to breed in captivity: the agouti produces four offspring per year [7] the lappe produces two offspring per year[8], and the capybara can produce eight offspring per year[9][10]. These animals are ideal in that they can utilize local feed resources and are adapted to local conditions of high heat and humidity. The meats produced by these rodents are highly nutritious, with high protein values and low fat and cholesterol concentration[11][12][13][14].
spp., also known as whipworms, have parasitized many domesticated species, causing enteritis, diarrhea, and weight loss [15].
spp. adults live in the caecum and colon; this predilection site has occurred due to evolution. The life cycle is direct; eggs with characteristic bi-polar plugs are passed in the feces and take two to three weeks to become infective (
) [16]. Animals become infected by the ingestion of infective eggs[16]. However, there has been limited information on the effects of
spp. on neotropical rodents (domestic and semi-domestic). Thus, the objective of this review is to summarize the species of
that parasitizes these rodents, the effect of this parasite on these animals, and the zoonotic potential of this pathogen.
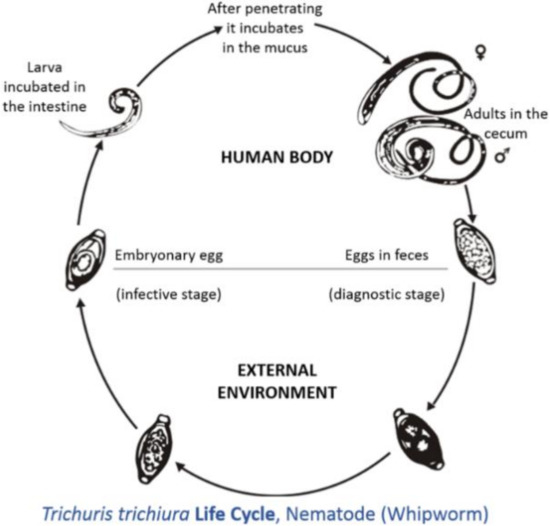
Life cycle of
(taken from[17]).
2. Trichuris spp. of Veterinary and Public Health Importance
2.1. Trichuriasis of Man
Trichuriasis is one of the major infectious diseases of children in developing countries[18].
is a major, soil-transmitted helminth targeted by the World Health Organization in their mass drug administration program for pre-school and primary school children in endemic developing countries[18]. There have been several cases of trichuriasis reported in humans. In some cases, it has been due to three
spp.:
,
, and
. Humans have been infected with
, and the diagnosis was made based on the morphology of the eggs and vulva from an adult female[19]. Molecular techniques were used on
spp. egg present in feces to identify
and
in human populations from Thailand[20].
has been experimentally given to humans, and the author stated that feces were negative for
eggs 40 days post-infection[21]. Experimentally treated patients showed no symptoms of gastrointestinal distress[21]. In contrast to the previous studies, Kradin et al.[22] showed that iatrogenic infection with
resulted in a persistent active infection in man. Pathological findings from colonic biopsies showed several round helminths beneath the ileocecal mucosa epithelium [22].
has human and non-human primates as its natural hosts[23]. Mixed infections with various
spp. in humans have been documented. There have been cases of mixed infections with
and
[24][25]. The identification of the species of
spp. was based on the morphology of eggs[24] and polymerase chain reactions of the helminth eggs [25].
and
was also found in the stool samples of dogs that roamed around the community. This shows that dogs are key to the transmission of
spp. to humans, but further work needs to be done to validate this finding[25].
Infections with
have been reported in children and adults [19][26][27]. However, all cases of trichuriasis in humans caused by
have had some association with dogs, and the diagnosis was made based on morphology of eggs present in the feces. Clinical signs reported in humans are abdominal discomfort, epigastric pain, nausea, vomiting, diarrhea, and poor appetite[24]. Patients with
[19] have been treated with mebendazole and albendazole with improvements of clinical signs [19][24][26][27]. However, in vivo studies on albendazole and mebendazole have shown little efficacy against
[28]. At 14 days post-treatment, there was no difference in the disease prevalence seen between treatments of patients with 400 grams of albendazole [28]. Therefore, alternative anthelmintic treatment against
should be investigated. Ivermectin has been used to treat
spp.; however, it is very ineffective, as these parasites have become resistant to this drug. However, due to the increased prevalence of anthelmintic resistance, the drugs used to treat trichuriasis should be done with caution.
2.2. Morphological and Molecular Identifications of Trichuris spp.
2.2.1. Morphological Identification of
2.2.1. Morphological Identification of
Trichuris spp. in Pigs, Dogs, Cats, Humans, and Non-Human Primates
spp. in Pigs, Dogs, Cats, Humans, and Non-Human Primates
Morphological analysis of
Trichurisspp. has been used for identification within various host species.
Trichuris trichiurainfection has been investigated in humans, non-human primates, and pigs, but based on morphological analysis, the
T. trichiura found in humans and non-human primates were indistinguishable [29]. In pigs,found in humans and non-human primates were indistinguishable[29]. In pigs,
T. suiswas differentiated from
T. trichiura, based on the lack of peri-cloacal papillae in adult specimens. In female specimens, there were no morphological differentiation between
T. suisand
T. trichiura [29]. Ruminants evaluated in India using morphological analysis identified[29]. Ruminants evaluated in India using morphological analysis identified
T. ovis as the major parasite [30].as the major parasite [30]. [
Further research was done in domestic cats in St. Kitts. Based on the size of the
Trichurisspp. identified, authors believed that it was
T. campanula, but based on the vulva structure the authors confirmed it was
T. serrata. In conclusion, the authors, identified the parasite as
T. serrata, but recommended that molecular studies must be done in order to reliably identify this parasite [31]. In dogs, male and female adult, but recommended that molecular studies must be done in order to reliably identify this parasite[31]. In dogs, male and female adult
T. vulpis could be identified based on nine parameters (including body length, length of cuticular processes, and width of body at tail part) [32]. Malecould be identified based on nine parameters (including body length, length of cuticular processes, and width of body at tail part)[32]. Male
T. vulpis can be distinguished from other species by spicule sheath ornamentation (the dimensions of the spicule) [32].can be distinguished from other species by spicule sheath ornamentation (the dimensions of the spicule) [32].
Recently, the morphometric approach analyzing the adult worms and eggs of
Trichuris spp. of non-human primates were analyzed [33,34]. Morphometric data on the adult worms showed that features present in the females made them indistinguishable for species characteristics, but adult male worms may be used to differentiatespp. of non-human primates were analyzed[33][34]. Morphometric data on the adult worms showed that features present in the females made them indistinguishable for species characteristics, but adult male worms may be used to differentiate
Trichuris populations [33]. Geometric morphometric analysis is a new diagnostic tool that can be used to differentialpopulations[33]. Geometric morphometric analysis is a new diagnostic tool that can be used to differential
Trichuris spp. present in non-human primates. However, further data must be collected to determine the sensitivity and specificity of this diagnostic tool [34]. Combination of various techniques, such as the use of molecular and morphological analysis, should be performed for confirmation of variousspp. present in non-human primates. However, further data must be collected to determine the sensitivity and specificity of this diagnostic tool[34]. Combination of various techniques, such as the use of molecular and morphological analysis, should be performed for confirmation of various
Trichuris spp. [33].spp. [33].
2.2.2. Molecular Identification of Trichuris spp. in Domestic and Non-Domestic Ruminants
Molecular techniques have been used to identify various
spp. in their animals or human hosts. Such techniques have been applied to
spp. found in ruminants (both domesticated and non-domesticated). Four
spp.—
,
,
and
—have been identified as inhabiting the caecum and colon of ruminants[35][36][37][38][39][40][41][42][43][44][45][46]. One of the major discoveries was the identification of
and
as the same species by isoenzymes [35], using second, internally transcribed spacer ribosomal DNA (ITS2 rDNA)[38] and ITS1-5.8S-1TS2 [37]. Further molecular analysis was done comparing
and
, where the entire mitochondrial DNA (mtDNA) was analyzed[42], and with the use of internally transcribed spacers 1, 2, and 16S, partial DNA sequencing (ITS1, 2, 16rDNA) was completed[44]. Based on mtDNA and rDNA,
and
can be classified as two different species.
, found in small ruminants (sheep and goats), was characterized using isoenzymes [36], ITS1-5.8S-1TS2 [37], and cytochrome oxidase subunit 1 and mitochondrial 16S rDNA [39]. Authors have stated that
is an independent species but has close relations to other
spp. that parasitize small ruminants.
has been identified in domestic ruminants with the use of molecular techniques; however, it was recently identified in wild ruminants, such as the roe deer (
), sika deer, (
), red deer (
), fallow deer (
), and mouflons (
)[43][44][45]. In wild ruminants,
was identified with use of ITS1-5.8S-1TS2[43][44][45], but in cattle different populations of
in Iran, Spain, and Japan were investigated using 16S partial gene mtDNA, as well as ITS1 and 2 [43]. Callejon et al. [43] noted that there were specific populations of
groups based on geographical location. The author noted that one reason may be due to two cryptic species of
from Japan and Iran, as well as another from Spain.
2.2.3. Molecular Identification of Trichuris spp. in Cats, Dogs, Pigs, Humans, and Non-Human Primates
spp. has also been identified molecularly in pets, such as dogs and cats. In cats it is associated with typhlitis, which also occurs in other animals[46]. Identification of
(cats) and
(dogs) was accomplished through the use of 18S rDNA (cats) and enzyme-linked immunosorbent assay (ELISA) and ITS1-5.8S-1TS2 (dogs)[47][48]. Comparative genetic studies were done of the
found in dogs and
found in pigs (wild and domesticated). There was a difference seen in amplified ITS1-5.8S-1TS2 rDNA between the
found in dogs and
found in pigs. Interestingly,
collected from wild pigs (
) and domestic pigs (
) showed no sequential genetic differences[49].
Several non-morphological processes were used to identify
found in pigs using isoenzymes[50], ITS 1 and ITS2 regions of rDNA [51], large mitochondrial subunits and ITS2[52], and nuclear ribosomes (18S, ITS2)[18]. Due to the zoonotic potential of
and its morphological similarity to
previous molecular studies have been done in both human and non-human primates[53][54][55].
spp. was taken from pigs (wild and domestic) and non-human primates (
and
) and analyzed by amplification of rDNA (ITS1-5.8S-1TS2). The authors confirmed that the
found in pigs was genetically different from
in
and
[53]. Nissen et al. [54] conducted a similar study to Cutillas et al.[53], but
and
were identified in pigs and humans in Uganda. The gastrointestinal tract of pigs only contained
, while in humans
,
, and a heterozygous type was identified [54]. This showed that the use of ITS 2 and β-tubulin allowed the identity of several species of
in humans to be highlighted.
The research done by Cutillas et al. [53]and Nissen et al.[54] highlights the fact that humans and non-human primates may be infected with several species of
that are generally classified as
This was seen with
spp. samples taken from the wild Japanese macaques (
), where the
spp. identified had genetic (18S rDNA) dissimilarity compared to those found in humans [56]. This new hypothesis sparked scientists to investigate this phenomenon at a molecular level (
). Ravasi et al.[57] investigated the genotype of human and non-human primates in Central Africa. Sequencing of the rDNA (ITS1-5.8S-1TS2) revealed two
genotypes that infect both humans and non-primates[57]. Ghai et al.[58] found similar results to Ravasi et al.[57], but three
genotypes were identified as circulating within human and non-human primates. Humans were infected with two genotypes: one genotype that was only common to human samples (Group 1), and another genotype that infected humans as well as non-human primates (black-and-white colobus (
), blue monkeys (
), grey-cheeked mangabeys (
), l’hoest monkeys (
), olive baboons (
), red colobus (
), red-tailed guenons (
), and the chimpanzee (
)) (Group 3). The intermediary group (Group 2) had a
genotype that affected non-human primates (black-and-white colobus (
and the red colobus (
)[58]. Furthermore, this new species of
was found in the Francois’ leaf monkey (
) and the
using mtDNA, rDNA, and morphometry[59][60].
2.2.4. Molecular Identification Trichuris spp. in Rodents
spp. has been found in domestic livestock and pets, but there are also species that are specific to rodents. The initial molecular research that was done on the
spp. present in rodents focused on European rodents [61].
was identified in Murid rodents in Europe with the use of rDNA (ITS1-5.8S-ITS2). It was found that two lineages had occurred, due to geographical distribution. One was found in northern Spain to Denmark, and the other in the Southern Europe (Croatia, Romania, and Turkey) [61]. In recent years, several new species of
have been found in Arvicolinae rodents using multi-local enzyme electrophoresis[62]and rDNA (ITS1-5.8S-ITS2)[63]. Further investigations were done in the phylogeographic analysis of
in Europe, using the mtDNA cytochrome subunit 1 gene (cox1) and rDNA (ITS1-5.8S-ITS2). Nuclear genetics (ITS1-5.8S-ITS2) suggest that
show two geographic and genetic lineages (Neoarctic and Palaearctic). Mitochondrial results gave further details into the Palaearctic region, giving three geographic and genetic lineages (Northern Europe, Southern and Eastern Europe, and Italy and France) [64].
Scientists also investigated
present in Sigmodontinae rodents in South America (Argentina). New species, such as
, were identified based on morphological analysis [65]. Another species that was identified morphologically was
[64]. Based on molecular characteristics, using ITS2 (rDNA), a new species named
was identified[66]. Molecular analysis using cox1 and mitochondrial cytochrome b (cob) on the
spp. found in Sigmodontinae rodents found three clades corresponding to three different species, which were
,
, and
)[67]. Further to this,
was identified in
(Cricetidae: Sigmodontinae) using morphological mitochondrial (cox1 and cob) and nuclear (ITS2) markers [68].
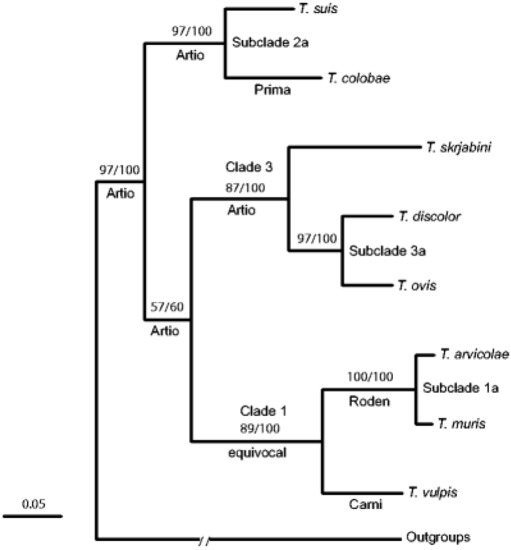
Callejon et al. [41][69] investigated nuclear (18S, triose phosphate isomerase) and mitochondrial (cox1, cob1) genes from
spp. from nine various host species
,
,
,
,
,
,
,
, and
) from Spain. The data show that
spp. could be divided in three clades: Clade 1 =
,
, and
; Clade 2 =
,
,
, and
. spp. ex
; Clade 3 =
,
, and
[69].
2.3. Immunomodulatory Effect of Trichuris spp.
spp. has been used in the treatment of gastrointestinal autoimmune diseases, such as inflammatory bowel disease, Crohn’s disease, and ulcerative colitis [70][71][72].
(pig whipworm) had been experimentally given to humans with no overt sign of gastrointestinal illness. The eggs produced from the feces remained constant, and only a low percentage of these eggs embryonated in vitro[21]. Some authors also noted that treatment of patients with inflammatory bowel disease, ulcerative colitis, and Crohn’s disease with
showed improvement in gastrointestinal signs, and in the management of disease the subjects were given ova every three weeks [70][71][72]. Surprisingly, Kradin et al.[22] noted that a patient that underwent treatment for Crohn’s disease using
had adult worms beneath the ileocecal mucosal epithelium. This case does raise concerns about persistent infection from
in man [22].
Further work was done on the use of excretory secretory products of
in rats[73]. The investigation of the use of excretory products of
in swine epithelium cells was used as a model to be used in humans. It was noted that the excretory secretory products (ESPs) elicited the production of interleukin (IL)-6 and IL-10, which have been identified as anti-inflammatory cytokines that inhibit Th-1 responses. This proved that ESPs from
have immunomodulatory effects and can be used as candidates in the treatment of inflammatory bowel disease[73]. The use of ESPs from
may be safer than the actual treatment with ova.
Subsequent research was done on the immunomodulatory and immunogenic effects of the proteins and ESPs of
and
[74][75][76]. Proteins were analyzed from adult worm extract and fragments of
. These extracts and fragments were placed in cell cultures of human peripheral blood monocytes, and elicited the production of IL-10, IL-12, and TNF-α. Some fractions showed the inhibition of IL-5 production. The downregulation of IL-5 is a feature of a Th-2 response[74]. Santos et al. [74] concluded that protein fractions of
can be used in the treatment and prevention of allergic and autoimmune diseases. Immunogenic research was also conducted on the ESPs of
, and specific immunogenic proteins were identified. The structure of one such protein was Tm16, which was characterized and could be used in the production of a vaccine[75]. Shears et al.[76] noted that ESPs from
elicited production of IL-9 and IL-13 when inoculated into rats. Eleven immunogenic proteins from the ESP of
were also identified, and these could be used in the production of a vaccine [76]. Recent studies show that there is tremendous potential for
in human autoimmune disease, as well as vaccine development in rural countries where trichuriasis infections are prevalent.
References
- Brown-Uddenberg, R.C.; Garcia, G.W.; Baptiste, Q.S.; Counand, T.; Adogwa, A.O.; Sampson, T. The Agouti (Dasyprocta leporina, D. aguti) Booklet and Producers’ Manual; GWG Publications: Champs Fleurs, Trinidad and Tobago, 2014. Available online: http://ostasp.brinkster.net/ (accessed on 17 June 2020).
- Jones, K.R.; Garcia, G.W. Gastrointestinal parasites of domesticated animals introduced into the Neo-tropics (New World Tropics). Concepts Dairy Vet. Sci. 2018, 1, 56–78.
- Jones, K.R.; Garcia, G.W. Endoparasites of domesticated animals that originated in the neo-tropics (new world tropics). Vet. Sci. 2019, 6, 24.
- Jones, K.R.; Lall, K.R.; Garcia, G.W. Endoparasites of selective native non-domesticated mammals in the neo-tropics (new world tropics). Vet. Sci. 2019, 6, 87.
- Paterson, R.T.; Joaquin, N.; Chamon, K.; Palomino, E. The productivity of small animal species in small-scale mixed farm-ing systems in subtropical Bolivia. Trop. Anim. Health Prod. 2001, 33, 1–14.
- Manjeli, Y.; Tchoumboue, J.; Njwe, R.M.; Teguia, A. Guinea-pig productivity under traditional management. Trop. Anim. Health Prod. 1998, 30, 115–122.
- Jones, K.R.; Garcia, G.W. Anthelmintic usage on the reproductive parameter in captive reared agoutis (Dasyprocta leporina) in Trindad and Tobago, West Indies. Trop. Agric. 2020, 97, In Press.
- Govoni, G.; Fielding, D. Paca (Agouti paca) and Agouti (Dasyprocta spp.)- Minilivestock Production in the Amazonas State of Venezuela: 1. Biology. Tropicultura 2001, 19, 56–60.
- Chapman, C.A. Biology of Capybaras. J. Mammal. 1991, 72, 206–208.
- Alvarez, M.R.; Kravetz, F.O. Reproductive performance of capybaras (Hyrochoerus hydrochaeris) in captivity under different management systems in Argentina. Anim. Res. 2006, 55, 153–164.
- Ali, A.J.; Jones, K.R. Nutritive value and physical properties of Neo-tropical rodent meat- with emphasis on the Capybara (Hydrochoerus hydrochaeris). Animals 2020, 10, 2134.
- Dalle Zotte, A. Perception of rabbit meat quality and major factors influencing the rabbit carcass and meat quality. Livest. Prod. Sci. 2002, 75, 11–32.
- Pla, M.; Pascual, M.; Arino, B. Protein, Fat and Moisture Content of Retail Cuts of Rabbit Meat Evaluated with the NIRS Methodology. World Rabbit. Sci. 2004, 12, 149–158.
- Nogueira-Filho, S.L.G.; Nogueira, S.S.C. Capybara meat: An extraordinary resource for food security in South America. Meat Sci. 2018, 145, 329–333.
- Soulsby, E.J. Helminths, Arthropods and Protozoa of Domesticated Animals, 6th Revised ed.; Baillière, Tindall & Cassell; The Amer-ican Society of Tropical Medicine and Hygiene: Arlington, VA, USA, 1968.
- Zajac, A.M.; Conboy, G.A. Veterinary Clinical Parasitology; John Wiley & Sons: Hoboken, NJ, USA, 2012.
- Life Cycle of Trichuris Found inside the Body. Available online: https://en.wikipedia.org/wiki/Trichuris_trichiura#/media/File:Trichuris_trichiura_Life_Cycle.tif (accessed on 7 January 2021).
- Albonico, M.; Allen, H.; Chitsulo, L.; Engels, D.; Gabrielli, A.F.; Savioli, L. Controlling soil-transmitted helminthiasis in pre-school-age children through preventive chemotherapy. PLoS Negl. Trop. Dis. 2008, 2, e126.
- Hall, J.E.; Sonnenberg, B. An apparent case of human infection with whip-worm of dogs, Trichuris vulpis (Froelich, 1789). J. Parasitol. 1956, 42, 197–199.
- Phosuk, I.; Sanpool, O.; Thanchomnang, T.; Sadaow, L.; Rodpai, R.; Anamnart, W.; Janwan, P.; Wijit, A.; Laymanivong, S.; Aung, W.P.P.; et al. Molecular identification of Trichuris suis and Trichuris trichiura eggs in human populations from Thai-land, Lao PDR, and Myanmar. Am. J. Trop. Med. Hyg. 2018, 98, 39–44.
- Beer, R.J. Experimental infection of man with pig whipworm. Br. Med. J. 1971, 2, 44.
- Kradin, R.L.; Badizadegan, K.; Auluck, P.; Korzenik, J.; Lauwers, G.Y. Iatrogenic Trichuris suis infection in a patient with Crohn disease. Arch. Pathol. Lab. Med. 2006, 130, 718–720.
- Sunkara, T.; Sharma, S.R.; Ofosu, A. Trichuris trichiura—An Unwelcome Surprise during Colonoscopy. Am. J. Trop. Med. Hyg. 2018, 99, 555.
- Vásquez, O.T.; Martínez, I.B.; Romero, R.C.; Valencia, S.R.; Tay, J.Z. Mixed infection by Trichuris trichiura and Trichuris vulpis. Rev. Gastroenterol. Peru Organo Soc. Gastroenterol. Peru 1997, 17, 255–258.
- Areekul, P.; Putaporntip, C.; Pattanawong, U.; Sitthicharoenchai, P.; Jongwutiwes, S. Trichuris vulpis and T. trichiura infec-tions among schoolchildren of a rural community in north-western Thailand: The possible role of dogs in disease transmis-sion. Asian Biomed. 2010, 4, 49–60.
- Dunn, J.J.; Columbus, S.T.; Aldeen, W.E.; Davis, M.; Carroll, K.C. Trichuris vulpis recovered from a patient with chronic diar-rhea and five dogs. J. Clin. Microbiol. 2002, 40, 2703–2704.
- Márquez-Navarro, A.; García-Bracamontes, G.; Álvarez-Fernández, B.E.; Ávila-Caballero, L.P.; Santos-Aranda, I.; Díaz-Chiguer, D.L.; Sánchez-Manzano, R.M.; Rodríguez-Bataz, E.; Nogueda-Torres, B. Trichuris vulpis (Froelich, 1789) infection in a child: A case report. Korean J. Parasitol. 2012, 50, 69–71.
- Olsen, A.; Namwanje, H.; Nejsum, P.; Roepstorff, A.; Thamsborg, S.M. Albendazole and mebendazole have low efficacy against Trichuris trichiura in school-age children in Kabale District, Uganda. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 443–446.
- Ooi, H.K.; Tenora, F.; Itoh, K.; Kamiya, M. Comparative study of Trichuris trichiura from non-human primates and from man, and their difference with T. suis. J. Vet. Med. Sci. 1993, 55, 363–366.
- Kuchai, J.A.; Ahmad, F.; Chishti, M.Z.; Dar, J.A.; Tak, H. On morphology and morphometry of Trichuris ovis Abildgaard, 1795 recovered from ruminants of Ladakh, India. J. Buffalo Sci. 2013, 2, 49–52.
- Ketzis, J.K. Trichuris spp. infecting domestic cats on St. Kitts: Identification based on size or vulvar structure? SpringerPlus 2015, 4, 115.
- Yevstafieva, V.А.; Kravchenko, S.O.; Gutyj, B.V.; Melnychuk, V.V.; Kovalenko, P.N.; Volovyk, L.B. Morphobiological analy-sis of Trichuris vulpis (Nematoda, Trichuridae), obtained from domestic dogs. Regul. Mech. Biosyst. 2019, 10, 165–171.
- García-Sánchez, A.M.; Rivero, J.; Callejón, R.; Zurita, A.; Reguera-Gomez, M.; Valero, M.A.; Cutillas, C. Differentiation of Trichuris species using a morphometric approach. Int. J. Parasitol. Parasites Wildl. 2019, 9, 218–223.
- García-Sánchez, A.M.; Reguera-Gomez, M.; Valero, M.A.; Cutillas, C. Differentiation of Trichuris species eggs from non-human primates by geometric morphometric analysis. Int. J. Parasitol. Parasites Wildl. 2020, 12, 214–219.
- Cutillas, C.; German, P.; Arias, P.; Guevara, D. Trichuris ovis and Trichuris globulosa: Morphological, biometrical, and genetic studies. Exp. Parasitol. 1995, 81, 621–625.
- Cutillas, C.; German, P.; Arias, P.; Guevara, D. Characterization of Trichuris skrjabini by isoenzyme gel electrophoresis: Comparative study with Trichuris ovis. Acta Trop. 1996, 62, 63–69.
- Cutillas, C.; Oliveros, R.; De Rojas, M.; Guevara, D.C. Determination of Trichuris skrjabini by sequencing of the ITS1–5.8 S–ITS2 segment of the ribosomal DNA: Comparative molecular study of different species of trichurids. J. Parasitol. 2004, 90, 648–652.
- Oliveros, R.; Cutillas, C.; De Rojas, M.; Arias, P. Characterization of four species of Trichuris (Nematoda: Enoplida) by their second internal transcribed spacer ribosomal DNA sequence. Parasitol. Res. 2000, 86, 1008–1013.
- Callejón, R.; De Rojas, M.; Ariza, C.; Ubeda, J.M.; Guevara, D.C.; Cutillas, C. Cytochrome oxidase subunit 1 and mitochon-drial 16S rDNA sequences of Trichuris skrjabini (Tricocephalida: Trichuridae). Parasitol. Res. 2009, 104, 715–716.
- Callejón, R.; Halajian, A.; De Rojas, M.; Marrugal, A.; Guevara, D.; Cutillas, C. 16S partial gene mitochondrial DNA and in-ternal transcribed spacers ribosomal DNA as differential markers of Trichuris discolor populations. Vet. Parasitol. 2012, 186, 350–363.
- Callejón, R.; Nadler, S.; De Rojas, M.; Zurita, A.; Petrášová, J.; Cutillas, C. Molecular characterization and phylogeny of whipworm nematodes inferred from DNA sequences of cox1 mtDNA and 18S rDNA. Parasitol. Res. 2013, 112, 3933–3949.
- Liu, G.H.; Wang, Y.; Xu, M.J.; Zhou, D.H.; Ye, Y.G.; Li, J.Y.; Song, H.Q.; Lin, R.Q.; Zhu, X.Q. Characterization of the complete mitochondrial genomes of two whipworms Trichuris ovis and Trichuris discolor (Nematoda: Trichuridae). Infect. Genet. Evol. 2012, 12, 1635–1641.
- Salaba, O.; Rylková, K.; Vadlejch, J.; Petrtýl, M.; Scháňková, Š.; Brožová, A.; Jankovská, I.; Jebavý, L.; Langrová, I. The first determination of Trichuris sp. from roe deer by amplification and sequenation of the ITS1-5.8 S-ITS2 segment of ribosomal DNA. Parasitol. Res. 2013, 112, 955–960.
- Vejl, P.; Nechybová, S.; Peřinková, P.; Melounová, M.; Sedláková, V.; Vašek, J.; Čílová, D.; Rylková, K.; Jankovská, I.; Va-dlejch, J.; et al. Reliable molecular differentiation of Trichuris ovis and Trichuris discolor from sheep (Ovis orientalis aries) and roe deer (Capreolus capreolus) and morphological characterisation of their females: Morphology does not work sufficiently. Parasitol. Res. 2017, 116, 2199–2210.
- Nechybová, S.; Vejl, P.; Hart, V.; Melounová, M.; Čílová, D.; Vašek, J.; Jankovská, I.; Vadlejch, J.; Langrová, I. Long-term occurrence of Trichuris species in wild ruminants in the Czech Republic. Parasitol. Res. 2018, 117, 1699–1708.
- Wulcan, J.M.; Ketzis, J.K.; Dennis, M.M. Typhlitis Associated with Natural Trichuris sp. Infection in Cats. Vet. Pathol. 2020, 57, 266–271.
- Ketzis, J.K.; Verma, A.; Burgess, G. Molecular characterization of Trichuris serrata. Parasitol. Res. 2015, 114, 1993–1995.
- Cutillas, C.; de Rojas, M.; Ariza, C.; Ubeda, J.M.; Guevara, D. Molecular identification of Trichuris vulpis and Trichuris suis isolated from different hosts. Parasitol. Res. 2007, 100, 383–389.
- Elsemore, D.A.; Geng, J.; Flynn, L.; Cruthers, L.; Lucio-Forster, A.; Bowman, D.D. Enzyme-linked immunosorbent assay for coproantigen detection of Trichuris vulpis in dogs. J. Vet. Diagn. Investig. 2014, 26, 404–411.
- Oliveros, R.; Cutillas, C.; Arias, P.; Guevara, D. Morphologic, biometric, and isoenzyme characterization of Trichuris suis. Parasitol. Res. 1998, 84, 513–515.
- Liu, G.H.; Zhou, W.; Nisbet, A.J.; Xu, M.J.; Zhou, D.H.; Zhao, G.H.; Wang, S.K.; Song, H.Q.; Lin, R.Q.; Zhu, X.Q. Characteriza-tion of Trichuris trichiura from humans and T. suis from pigs in China using internal transcribed spacers of nuclear riboso-mal DNA. J. Helminthol. 2014, 88, 64–68.
- Muramatsu, R.; Sato, R.; Onuma, N.; Sasai, K.; Shibahara, T.; Matsubayashi, M. Molecular Identification of Trichuris suis Worms and Eggs in Pig Feces, Infected Intestines, and Farm Environments in Japan. Jpn. Agric. Res. Q. JARQ 2020, 54, 271–275.
- Cutillas, C.; Callejon, R.; De Rojas, M.; Tewes, B.; Ubeda, J.M.; Ariza, C.; Guevara, D.C. Trichuris suis and Trichuris trichiura are different nematode species. Acta Trop. 2009, 111, 299–307.
- Nissen, S.; Al-Juburry, A.; Hansen, V.A.; Olsen, A.; Christensen, H.; Thamsborg, S.M.; Nejsum, P. Genetic analysis of Trichu-ris suis and Trichuris trichiura recovered from humans and pigs in a sympatric setting in Uganda. Vet. Parasitol. 2012, 188, 68–77.
- Liu, G.H.; Gasser, R.B.; Su, A.; Nejsum, P.; Peng, L.; Lin, R.Q.; Li, M.W.; Xu, M.J.; Zhu, X.Q. Clear genetic distinctiveness be-tween human-and pig-derived Trichuris based on analyses of mitochondrial datasets. PLoS Negl. Trop. Dis. 2012, 6, e1539.
- Arizono, N.; Yamada, M.; Tegoshi, T.; Onishi, K. Molecular identification of Oesophagostomum and Trichuris eggs isolated from wild Japanese macaques. Korean J. Parasitol. 2012, 50, 253–257.
- Ravasi, D.F.; O’Riain, M.J.; Davids, F.; Illing, N. Phylogenetic evidence that two distinct Trichuris genotypes infect both hu-mans and non-human primates. PLoS ONE 2012, 7, e44187.
- Ghai, R.R.; Simons, N.D.; Chapman, C.A.; Omeja, P.A.; Davies, T.J.; Ting, N.; Goldberg, T.L. Hidden population structure and cross-species transmission of whipworms (Trichuris sp.) in humans and non-human primates in Uganda. PLoS Negl. Trop. Dis. 2014, 8, e3256.
- Liu, G.H.; Gasser, R.B.; Nejsum, P.; Wang, Y.; Chen, Q.; Song, H.Q.; Zhu, X.Q. Mitochondrial and nuclear ribosomal DNA evidence supports the existence of a new Trichuris species in the endangered françois’ leaf-monkey. PLoS ONE 2013, 8, e 66249.
- Cutillas, C.; de Rojas, M.; Zurita, A.; Oliveros, R.; Callejón, R. Trichuris colobae n. sp. (Nematoda: Trichuridae), a new species of Trichuris from Colobus guereza kikuyensis. Parasitol. Res. 2014, 113, 2725–2732.
- Callejón, R.; de Rojas, M.; Nieberding, C.; Foronda, P.; Feliú, C.; Guevara, D.; Cutillas, C. Molecular evolution of Trichuris muris isolated from different Muridae hosts in Europe. Parasitol. Res. 2010, 107, 631–641.
- Feliu, C.; Spakulová, M.; Casanova, J.C.; Renaud, F.; Morand, S.; Hugot, J.P.; Santalla, F.; Durand, P. Genetic and morpholog-ical heterogeneity in small rodent whipworms in southwestern Europe: Characterization of Trichuris muris and description of Trichuris arvicolae n. sp. (Nematoda: Trichuridae). J. Parasitol. 2000, 86, 442–449.
- Cutillas, C.; Oliveros, R.; De Rojas, M.; Guevara, D.C. Determination of Trichuris muris from murid hosts and T. arvicolae (Nematoda) from arvicolid rodents by amplification and sequentiation of the ITS1–5.8 S-ITS2 segment of the ribosomal DNA. Parasitol. Res. 2002, 88, 574–582.
- Callejon, R.; de Rojas, M.; Feliu, C.; Balao, F.; Marrugal, A.; Henttonen, H.; Guevara, D.; Cutillas, C. Phylogeography of Tri-churis populations isolated from different Cricetidae rodents. Parasitology 2012, 139, 1795–1813.
- Robles, M.D.R. New species of Trichuris (Nematoda: Trichuridae) from Akodon montensis Thomas, 1913, of the Paranaense forest in Argentina. J. Parasitol. 2011, 97, 319–327.
- Robles, M.D.R.; Cutillas, C.; Panei, C.J.; Callejón, R. Morphological and molecular characterization of a new Trichuris spe-cies (Nematoda-Trichuridae), and phylogenetic relationships of Trichuris species of cricetid rodents from Argentina. PLoS ONE 2014, 9, e112069.
- Callejón, R.; Robles, M.D.R.; Panei, C.J.; Cutillas, C. Molecular diversification of Trichuris spp. from Sigmodontinae (Cri-cetidae) rodents from Argentina based on mitochondrial DNA sequences. Parasitol. Res. 2016, 115, 2933–2945.
- Robles, M.D.R.; Cutillas, C.; Callejón, R. Morphological-molecular characterization and phylogenetic relationships of a new Trichuris species (Nematoda: Trichuridae) parasitic on Holochilus chacarius (Cricetidae: Sigmodontinae) from the Chaco ecoregion (Argentina). Infect. Genet. Evol. 2018, 58, 66–76.
- Callejón, R.; Cutillas, C.; Nadler, S.A. Nuclear and mitochondrial genes for inferring Trichuris phylogeny. Parasitol. Res. 2015, 114, 4591–4599.
- Summers, R.W.; Elliott, D.E.; Qadir, K.; Urban, J.F., Jr.; Thompson, R.; Weinstock, J.V. Trichuris suis seems to be safe and pos-sibly effective in the treatment of inflammatory bowel disease. Am. J. Gastroenterol. 2003, 98, 2034–2041.
- Summers, R.W.; Elliott, D.E.; Urban, J.F.; Thompson, R.; Weinstock, J.V. Trichuris suis therapy in Crohn’s disease. Gut 2005, 54, 87–90.
- Summers, R.W.; Elliott, D.E.; Urban, J.F., Jr.; Thompson, R.A.; Weinstock, J.V. Trichuris suis therapy for active ulcerative coli-tis: A randomized controlled trial. Gastroenterology 2005, 128, 825–832.
- Parthasarathy, G.; Mansfield, L.S. Trichuris suis excretory secretory products (ESP) elicit interleukin-6 (IL-6) and IL-10 secre-tion from intestinal epithelial cells (IPEC-1). Vet. Parasitol. 2005, 131, 317–324.
- Santos, L.N.; Gallo, M.B.; Silva, E.S.; Figueiredo, C.A.V.; Cooper, P.J.; Barreto, M.L.; Loureiro, S.; Pontes‐de‐Carvalho, L.C.; Alcantara‐Neves, N.M. A proteomic approach to identify proteins from Trichuris trichiura extract with immunomodulatory effects. Parasite Immunol. 2013, 35, 188–193.
- Liu, Z.; Kelleher, A.; Tabb, S.; Wei, J.; Pollet, J.; Hotez, P.J.; Bottazzi, M.E.; Zhan, B.; Asojo, O.A. Identification, Characteriza-tion, and Structure of Tm16 from Trichuris muris. J. Parasitol. Res. 2017, 2017, 4342789.
- Shears, R.K.; Bancroft, A.J.; Sharpe, C.; Grencis, R.K.; Thornton, D. Vaccination against whipworm: Identification of potential immunogenic proteins in Trichuris muris excretory/secretory material. Sci. Rep. 2018, 8, 1–10.
