Marine mammals are sentinels for the marine ecosystem and threatened by numerous factors including infectious diseases. One of the most frequently isolated bacteria are beta-hemolytic streptococci. However, knowledge on ecology and epidemiology of streptococcal species in marine mammals is very limited.
- streptococci
- infectious diseases
- marine mammals
1. Streptococcal Findings in Marine Mammals
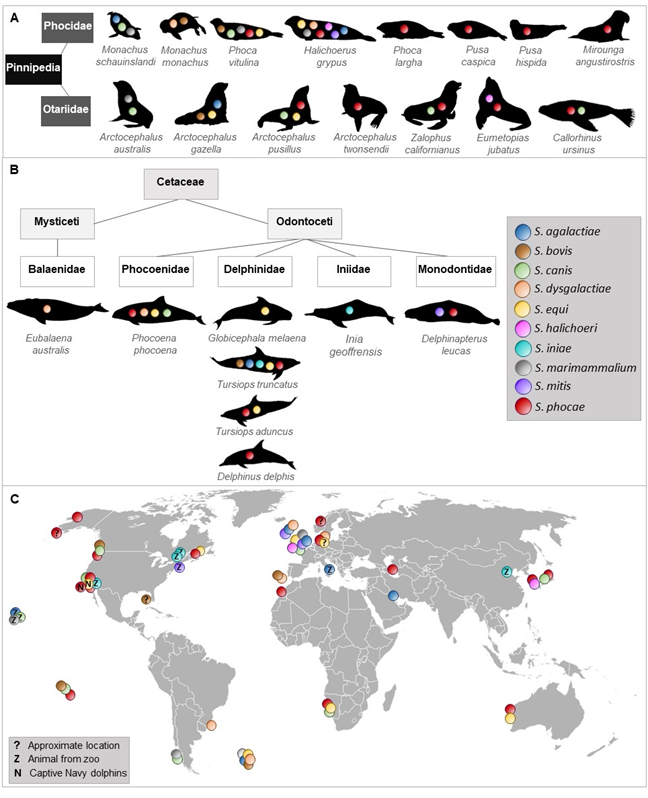
To the best of our knowledge, 10 streptococcal species were isolated and identified more than once from 23 species of Pinnipedia and Cetaceae worldwide (Figure 1).
Figure 1. Occurrence of streptococcal species described in different marine mammals. Streptococcal species that have been isolated and identified at least twice in pinnipeds (A) and cetaceans (B). (C) shows a world map indicating location of streptococcal species detected in marine mammals.
2. Streptococcus Agalactiae
S. agalactiae, also known as a bovine and human pathogen [1][2][3][59–61], was isolated from a wound and navel infection of grey seals (Halichoerus grypus) in North Rona in 1985 and 1986 [4][62] and from lesions of fight wounds, pneumonia, lymphadenitis as well as from lung and spleen samples of Antarctic fur seals (Arctocephalus gazella) from 1984–1987 on Bird Island, South Georgia [5][63]. Later, it was isolated from lesions and visceral organs (liver and lung) of a captive common bottlenose dolphin (Tursiops truncatus) that suffered from fatal necrotizing fasciitis and myositis [6][64]. One year later, the isolation of S. agalactiae from epaxial muscles of a wild stranded bottlenose dolphin was reported [7][65]. This strain caused 90% mortalities in tilapia in experimental infections and showed high similarity with strains associated with mullet kill in the concurrent Kuwait Bay. A mullet was found in the stomach of the dead dolphin, which might have served as a possible way of transmission. A study of human S. agalactiae strains from fish, seals, a dolphin, and a frog indicated zoonotic and anthroponotic hazard by causing severe disease in fish and compromising food security [8][66]. Between 2012 and 2014, S. agalactiae was isolated from a stranded grey seal on the British coast with ocular pathology[9] [67]. In the Waikiki Aquarium, Honolulu, Haiwaii, S. agalactiae was isolated from two male healthy Hawaiian monk seals (M. schauinslandi) as part of their aerobic bacterial flora in the nasal cavity [10][68].
S. agalactiae is also known as serious fish pathogen [11][12][13][69–71]. In Brazil, high virulent strains were isolated from diseased Nile tilapia and transmission occurred by direct contact or through water [12][70]. Infection experiments confirmed the disease and revealed low LD50 for Nile tilapia. However, isolates from cattle did not cause any clinical signs in Nile tilapia and channel catfish indicating host specification and adaptation [14][72]. Human and bovine strains of S. agalactiae were able to cause disease in Nile tilapia, although there was no genetic relatedness of strains from fish, bovine, and human origin [15][73]. This suggests that the ability to cross host-specific barrier is not necessarily reflected by genetic linkage. Virulence gene profiling of S. agalctiae isolated from diseased tilapia in Thailand revealed a positive correlation of virulence genes content and pathogenicity [16][74]. Virulence genes for adhesion, invasion, and immune evasion were identified. Another study demonstrated that there were fish-specific genes or loci that were associated with disease in fish, while strains missing these regions were not able to cause morbidity in tilapia [17][75]. In addition, these fish-specific genes were mainly clustered in regions with signatures of mobile genetic elements and one fish-specific gene was found in the region, where the virulence genes rib or bca are in the human strain indicating genetic adaptation to the fish host.
3. Streptococcus Bovis
S. bovis has been isolated from the gastrointestinal tract and feces of cattle, sheep, goats [18][76], and dogs [19][77]. It has also been identified as human pathogen associated with endocarditis [20][78], meningitis [21][79], septic arthritis [22][80], bacteremia, and gastrointestinal disease [23][81]. Virulence factors associated with S. bovis infection were, for instance, extracellular proteins [24][82] and antigens [25][83]. S. bovis was detected in fur seals with pneumonia that was characterized by extensive polymorphonuclear infiltrations and necrosis or very widespread abscess formation and, frequently, by additionally fibrinous exudative pleurisy [5][63]. Together with S. phocae and S. canis it was also isolated from dead herpesvirus-positive harbor seal pups at the Smith Island, Washington [26][32]. A monk seal pup (Monachus monachus) found on the island Deserta Grande, Portugal died due to septicemia and S. bovis was isolated and considered as a potential causative agent [27][84]. In 2006, S. bovis isolation (together with S. equisimilis/mitis) from free-ranging bottlenose dolphins that were captured, sampled, and released in coastal Gulf of Mexico and Atlantic ocean waters was reported [28][85].
4. Streptococcus Canis
S. canis was first isolated from cows with mastitis and from dogs with different pathological findings [29][86], but can also cause infections in humans [30][31][32][56,57,87]. It has also been isolated from minks [33][88], feral cats [34][89] and cats [35][90]. Its virulence and pathogenicity were confirmed by the presence of virulence genes such as for fibronectin-binding protein, M proteins, protective antigen, and streptolysin [36][37][38][39][91–94]. The M protein of S. canis has also a high-affinity immunoglobulin G binding activity, which is not species-specific and facilitate S. canis to interact with different hosts [40][95].
A beta-hemolytic Streptococcus sp., biochemically similar to S. canis, was cultured from pyogranulomatus lesion of the laryngeal cartilages and epiglottis of an adult harbor seal (Phoca vitulina) [41][27]. S. canis was also isolated from peritoneal effusion of a captive California sea lion (Zalophus californianus) of the US Navy’s marine mammal program[42] [96] and from a California sea lion with bilateral corneal ulceration of the London Zoo, UK [43][97]. During an increased mortality among South American fur seal (Arctocephalus australis) pups on Guafo Island, Chile, South America, S. canis together with S. marimammalium were isolated and associated with moderate to marked, multifocal, mucopurulent bronchopneumonia [44][98]. In August 1994, S. canis (and also S. phocae, S. equi subsp. zooepidemicus) was isolated from spleen, liver, and kidney of Cape fur seals (Arctocephalus pusillus pusillus) at Cape Cross, Namibia that suffered from respiratory infections and abortions associated with starvation [45][99]. Seven cases of stranded harbor porpoises (Phocoena phocoena) in England and Wales between 1990 and 1996 had a S. canis septicemia, which was isolated from lungs with pulmonary abscesses and enlarged pulmonary associated lymph nodes [46][100]. S. canis (together with S. phocae) was cultured from blood and lung samples of a dead, stranded Northern fur seal (Callorhinus ursinus) with necrotizing and fibrinous pneumonia infiltrated by band neutrophils with intraluminal abscess of bronchi at the coast of Niigata, Japan [47][46]. In 2005, the isolation of S. canis from two dead harbor seal pups on Smith Island, Washington was reported [26][48][32,101]. One died from omphalophlebitis and the other from omphalitis with subsequent peritonitis. S. canis was also isolated from the oral cavity of a male, healthy Hawaiian monk seal of the Waikiki Aquarium, Honolulu, Hawaii [10][68].
5. Streptococcus Dysgalactiae
S. dysgalactiae subsp. equisimilis, previously known as S. equisimilis [49][102] and found in humans [50][103] and different animals such as dogs, cows, pigs, and horses [51][104], was isolated from Antarctic fur seal pups with septicemia and rhinitis in South Georgia, UK between 1979–1982 [52][105] and 1986 from a grey seal cow on North Rona, Scotland [4][62]. In the years 1988 and 1989, an increased number of harbor porpoise carcasses was observed in North and Baltic Seas [53][36]. Thirty-five isolates of beta-hemolytic streptococci were classified in Lancefield group L and identified as S. dysgalactiae subsp. dysgalactiae. In 1997, S. dysgalactiae and S. dysgalactiae subsp. equisimilis isolates were found in a dead, wild monk seal pup in association with a septicemia on the island Deserta Grande, Portugal [27][84]. Three isolates identified as S. dysgalactiae subsp. dysgalactiae were obtained from phocid seals (harbor and grey seals) stranded in the North and Baltic Seas between 1995 and 2000 [54][106]. Between 2005–2011, pathologic and microbiological findings of a southern right whale (Eubalaena australis) calf from Brazil indicated that beta-hemolytic S. dysgalactiae septicemia was responsible for the death of the animal [55][107].
6. Streptococcus Equi
S. equi causes infections in horses [56][108] and was associated with canine infectious respiratory disease [57][109]. A systemic infection with S. equi in a horse handler has also been reported [58][110]. Further studies confirmed the zoonotic potential of S. equi [59][60][53,111]. In November 1978, a female North Atlantic pilot whale (Globicephala melaena) suffering from bronchopneumonia with lesions stranded on Metis Beach, Canada and S. equi (no further identification) was isolated from lung parenchyma, pharynx, and pericardial fluid [61][35]. A study from 1980 reported the isolation of S. equi subsp. zooepidemicus (previously S. zooepidemicus) from grey seals associated with mild, purulent pneumonia [62][49]. In 1994, it was isolated from the conjunctiva and trachea of two adult female Cape fur seals that had septicemic S. phocae in Namibia [45][99]. A total of 32 beta-hemolytic streptococcal isolates, collected during the phocine distemper outbreak in 2002 from 28 different harbor seals of the German North Sea, were identified as S. equi subsp. zooepidemicus [63][112]. Later, the same scientists isolated the same or at least very close related strains of S. equi subsp. zooepidemicus from grey seals and other harbor seals [64][113]. A retrospective study on 42 dead bottlenose dolphins from the US Navy Marine Mammal Program during 1980-2010 demonstrated an association of the isolation of S. equi subsp. zooepidemicus with pneumonia [65][114]. 16S rRNA sequences for S. equi (and S. phocae) were found in blow samples collected from four wild healthy Indo-Pacific bottlenose dolphins (T. aduncus) in Shark Bay (SB), Western Australia, in 2012 [66][115].
7. Streptococcus Halichoeri
S. halichoeri, characterized as non-hemolytic and classified in Lancefield group B, was first isolated from dead grey seals in Iverness and Cornwall, UK [67][50] and few years later, in 2012, also from the kidney of a stranded, female Stellar sea lion (Eumetopias jubatus) in South Korea [68][116]. Also, in 2012, a severe case of a human infection with S. halichoeri was reported [69][117]. The patient had no contact to seals, but to fish, which could have been a possible transmission route. However, this was not tested. Another human infection was reported in 2018, where a man suffered from skin cellulitis due to S. halichoeri [70][118]. Shewmaker et al. [71][119] compared human and seal strains and concluded two subspecies S. halichoeri subsp. halichoeri for the seal isolates and S. halichoeri subsp. hominis for strains associated with human infections. The core genome of 20 S. halichoeri isolates from different hosts including dogs and minks contained 19 different streptococcal virulence factors, of which most were associated with adherence followed by proteases and toxins emphasizing its pathogenic potential [72][73][120,121].
8. Streptococcus Iniae
S. niae was described as new species in 1976, when it was first isolated from a captive Amazon freshwater dolphin (Inia geoffrensis) suffering from a dermatologic syndrome called “golf ball disease” in the Steinhart Aquarium, San Francisco, USA [74][122]. Further isolates were obtained from captive freshwater dolphins housed at the Niagara Falls Aquarium in New York, USA two years later [[75]123], and in 1983 from a captive Amazon River dolphin at the Pittsburgh Zoo, USA that also developed the “golf ball disease”[76] [124]. In 2015, a common dolphin died due to bacterial septicemia at Beijing aquarium, China, where S. iniae was isolated from hilar lymph nodes and pancreas of the dolphin [77][125].
S. iniae is also a serious fish pathogen [78][79][58,126]. Virulence mechanisms include a capsule with antiphagocytic function [80][127], the cytotoxin ß-hemolysin streptolysin S [81][128], an extracellular nuclease and s secreted nucleotidase that play an important role in immune evasion [82][129], a polysaccharide deacetylase involved in adherence, invasion, lysozyme resistance and survival in fish blood [83][130], and M-like protein [84][131]. Comparative genomics revealed genetic differences between strains from different hosts including I. geoffrensis and identified two plasticity zones that reflect adaptation to specific host environments [85][132]. Furthermore, the dolphin isolates differed from the fish and human isolates in lacking a capsule, forming denser and thicker biofilms, increased ability to withstand oxidative stress and were genetically highly divergent to the other isolates [86][133]. In addition, there were conserved mutation rates and mismatch/oxidized-guanine repair systems within phylogenetic clades, but significant differences between major phylogenetic lineages. Mutators might facilitate adaptation to novel hosts including immune escape. This indicates that S. iniae has the genetic repertoire to adapt very well to many different hosts.
9. Streptococcus Marimammalium
S. marimammalium was first isolated from the lung of a dead harbor seal and a dead grey seal in Iverness, Scotland [87][51]. In 2007/2008, it was also isolated (together with S. agalactiae and S. canis) from nasal and oral swabs of two healthy Hawaiian monk seals from the Waikiki Aquarium, Honolulu, Hawaii [10][68]. In 2016, it was also isolated from South American Fur Seal Pups with moderate to marked, multifocal, mucopurulent bronchopneumonia on Guafo Island, Chile, South America [44][98]. To our knowledge, nothing is known about virulence factors and pathogenicity of S. marimammalium.
10. Streptococcus Mitis
S. mitis is mainly known as member of the human oral cavity [88][89][134,135] and as opportunistic pathogen causing endocarditis and bloodstream infections in neutropenic and immunocompromised patients [90][91][92][136–138]. It is closely related to the human pathogen S. pneumoniae and its genome contain virulence genes involved in colonization and adherence, which might also be important for commensals to interact with host cells [93][139]. However, genes for hyaluronidase and capsular genes were absent.
S. mitis was isolated in 1985 from a blowhole swab of a captive, healthy white whale (Delphinapterus leucas) 139 days after captivity at the Mystic Marinelife Aquarium Connecticut, USA [94][140]. In 1985, it was isolated from lesions of a grey seal with peritonitis in North Rona, Scotland [4][62] and between 2012–2014 from clinically normal eyes of two grey seals stranded on the British coast [9][67]. These findings suggest that S. mitis might also be a commensal in some marine mammals. The commensalism and pathogenesis of S. mitis is reviewed by Mitchel, 2011 [95][141].
11. Streptococcus Phocae
S. phocae was first isolated and identified from lung, liver, spleen, and kidney samples of harbor seals suffering from pneumonia with areas of consolidation, purulent exudate in the bronchi, interlobular edema, and emphysema during a phocine distemper virus outbreak in northwestern Europe [34]. Later, S. phocae was also isolated from diseased Atlantic salmon[96][97] [142,143], stranded southern sea otters [98][144], and as gut commensal of Indian white shrimp [99][145]. Two subspecies are described, S. phocae subsp. salmonis for isolates from Atlantic salmon and S. phocae subsp. phocae for isolates from seals [100][146].
In August 1994, beta-hemolytic streptococci with high similarity to S. phocae were isolated from spleen, liver, and kidney of Cape fur seals at Cape Cross, Namibia that suffered from respiratory infections and abortions [45][99]. A total of 69 S. phocae isolates were obtained from harbor and grey seals from the North and the Baltic Sea investigated between 1995 and 2000 [54][106]. A study on phocid seals (harbor and grey seals) that were older than 19 months from the North Sea of Schleswig-Holstein, Germany reported two S. phocae isolates from intestines of phocid seals with intestinal displacements [101][147]. During diagnostic evaluation by the Animal Health Center, Abbotsford, British Columbia, Canada S. phocae was isolated from harbor seals with an increase of prevalence since 2000, ringed seal (P. hispida) pups from arctic Canada and two stranded harbor porpoises from Washington State [102][48]. In spring and summer 2000, more than 10,000 Caspian seals (Pusa caspica) were found dead with canine distemper virus infection as primary diagnosis [103][47]. The investigated animals suffered from broncho-interstitial pneumonia, lymphocytic necrosis and depletion in lymphoid organs, and the presence of typical intracytoplasmic inclusion bodies in multiple epithelia. S. phocae was isolated from three of eight animals. Between 2001 and 2003, vaginal and preputial swabs of California Sea Lions were collected for investigations of genital bacterial infections and urogenital carcinoma [104][37]. S. phocae was isolated from three specimen of cancer and three specimens of non-cancer animals stranded along the central and northern California coast. In November 2007, a short-beaked common dolphin (Delphinus delphis) stranded at La Graciosa, Canary Islands [105][45]. Diagnostic evaluation revealed bacterial septicemia, fibrino-necrotizing to pyogranulomatous dermatitis and panniculitis, embolic pneumonia, neutrophilic and lymphoplasmacytic meningo-choroiditis, random neutrophilic hepatitis, lymphoplasmacytic myocarditis and epicarditis, necrotizing adrenalitis, suppurative endometritis, and multicentric reactive lymphadenopathy cutaneous purulent nodules in the tail fluke, vegetative mitral valve endocarditis, and presumed postpartum pyometra. S. phocae could be cultured from lung, brain, and adrenal gland tissue. Morbillivirus was detected in the epithelium of the choroid plexus of the fourth ventricle. In November 2009, a female spotted seal (Phoca largha) stranded at Kotzebue Sound, Alaska and was diagnosed with pyometra [106][148]. S. phocae was isolated from the purulent discharge in uterine contents. Three Navy bottlenose dolphins (T. truncatus) developed in the time between 2009 and 2010 a strangles-like syndrome associated with S. phocae, which was isolated after the animals showed clinical signs such as inflammatory hemogram, neutrophilic leukocytosis, and unilateral cervical lymphadenopathy [107][149]. Between 2004 to 2010 S. phocae could be isolated from five harbor seal pups of the Smith Island in Washington, USA that were also tested positive for phocine herpes virus [26] [32]. S. phocae was also isolated from five cases of bacterial septicemia of white whales stranded in St. Lawrence Estuary between 1983 to 2012 [108][33]. Necropsy of a total of 241 harbor porpoises stranded at the eastern Pacific and western Atlantic coasts of Canada between 1988 to 2011 revealed bacterial septicemia with S. phocae isolation [109][150]. In winter 2012, an adult female Stellar sea lion stranded in South Korea and S. phocae was cultured from the liver [68][116]. The cause of death was unknown. During 85 postmortem investigations of marine mammals of the northeastern Pacific and arctic Canada stranded between 2007–2012 resulted in S. phocae isolates from harbor seals (n = 61), ringed seals (n = 5), harbor porpoises (n = 5), California sea lion (n = 7), Stellar sea lion (n = 3), Guadalupe fur seal (Arctocephalus twonsendii, n = 1) and elephant seal (Mirounga angustirostris, n = 1) [110][151]. Sequencing of 16S rRNA V4 hyper variable regions of blow samples collected from four wild healthy Indo-Pacific bottlenose dolphins (T. aduncus) in Shark Bay (SB), Western Australia, in 2012 identified S. phocae (and S. equi) [66][115]. In February 2014, S. phocae was isolated from a carcass of a subadult male northern seal at the coast of Niigata, Japan that suffered from necrotizing and fibrinous pneumonia with diffuse abscesses of all lung lobes and massive necrosis of kidney and liver [47][46]. Between 2010 to 2017 the health of captive and stranded Alaskan ice seals were investigated and S. phocae isolates were obtained from blood, abscess, and lymph node samples from ringed seals [111][152]. Harbor seals stranded at the coast of San Juan County, Washington, USA between 2002 to 2018 were examined and from one adult female animal a fatal septicemia caused by S. phocae was reported [112][153].
While the presence of an antiphagocytic capsule and virulence of S. phocae subsp. salmonis to Atlantic salmon has been demonstrated in infectivity experiments [96][113][114][142,154,155], whole genome analyses of S. phocae subsp. phocae identified typical streptococcal virulence factors such as fibronectin-binding proteins, the toxin streptolysin S and genes encoding for a capsule [115][156]. Invasion of fish and mammalian cell lines by S. phocae subsp. phocae has also been shown and confirmed its pathogenic potential [113][154].
However, S. phocae subsp. phocae also seems to be a commensal of the oral cavity of grey seals as revealed by microbiome analyses and 16S rRNA sequencing. A transmission of S. phocae to harbor porpoises via bites is also indicated [116][157] and S. phocae might be an opportunistic pathogen, at least for seals.
12. Streptococcus Viridans Group
In very few studies, streptococci isolated from marine mammals were identified as members of the S. viridans group (viridans streptococci), which includes streptococci that are usually alpha-hemolytic and inhabit the oral cavity, intestinal, and vaginal tract [117][118][119][158–160]. This group is very heterogeneous and includes species such as S. anginosus, S. mitis, S. sanguinis, S. mutans, and S. salivarius, which can also cause endocarditis [120][161], bacteremia [121][162], and respiratory infections [122][163].
Viridans streptococci were isolated from superficial abscesses, wounds, ocular and urethral discharges, and umbilici of live and from lung and liver samples of dead elephant seals, California sea lions and harbor seals stranded between January 1994 and December 1995 along the California Coast [123][164]. Viridans streptococci were isolated in mixed cultures with Arcanobacterium phocae from California sea lions, harbor seals, Northern elephant seals, sea otter and common dolphin stranded along the central California coast between 1994 and 2000 [124][165]. In Beluga whales that stranded at Cook Inlet (Alaska, USA) between 1998 and 2013 an isolate was identified as member of the S. viridans group [125][166]. Also, viridans streptococci were isolated from gastric fluid samples of free-ranging bottlenose dolphins from the southeastern United States during a catch and release health assessment between 2003 to 2005 [126][167].
13. One-Time only Detections of Streptococcal Species from Marine Mammals
In studies described above, streptococcal species have been isolated and identified at least twice or more. In the following, reports on one-time only descriptions of streptococcal species are summarized.
S. uberis was found in dead free-ranging male Antarctic fur seals with pneumonia and extensive polymorphonuclear infiltrations and necrosis or very widespread abscess formation and frequently there was an associated fibrinous exudative pleurisy [5][63]. S. oralis was isolated and identified by API strips from three swabs taken from healthy eyes of free-ranging grey seals stranded on the British coast between November 2012 and February 2014 [9][67]. In a metagenome dataset of blood, muscle, and fecal samples of a living stranded sperm whale (Physeter catodon) S. anginosus, S. pneumoniae, and S. suis were detected in blood and fecal samples, but not in the muscles [127][168]. The animal died 79 h after rescue. S. intermedius was detected in blow samples of free-ranging and presumably healthy grey whales from Magdalena Bay and the Gulf of California by polymerase chain reaction [128][169].