The fascia can be defined as a dynamic highly complex connective tissue network composed of different types of cells embedded in the extracellular matrix and nervous fibers: each component plays a specific role in the fascial system changing and responding to stimuli in different ways.
- fascia
- cells
- extracellular matrix
- nerve
1. Introduction
Researchers and clinicians have dedicated quite a bit of attention to fascial tissues over the last few decades; currently, their interest has been focused on their anatomical and pathophysiological features. Ultrasound (US) [1][2][3][4][5][1,2,3,4,5] and magnetic resonance imaging (MRI) [6] have taken important steps forward that permit us to study the dynamic structure and the alterations of the tissue. While in vivo studies are critical for analyzing some fascial properties, such as stiffness, thickness, gliding and relationships with other anatomical structures, the fasciae also need to be studied from a microscopic point of view.
Many morphological descriptions of the fasciae can be found in the literature. According to some authors [7][8][7,8], the deep muscular fascia consists of a dense, regular connective tissue similar to aponeurosis, characterized by extremely ordinate, parallel bundles of inelastic collagen fibers. Another definition that can be found in the same edition of Gray’s Anatomy [8] indicates that it is loose irregular connective tissue. According to others [9][10][9,10], it is formed by numerous laminae of dense connective tissue in which the collagen fibers can be aligned in more than one direction. For yet others [11], the laminae are difficult to distinguish because the collagen fibers often pass from one lamina to an adjacent one. Additionally, according to some investigators [12], the deep/muscular fasciae of the lower limbs are composed of intertwined bundles of collagen fibers.
Some investigators [13][14][15][13,14,15] have distinguished between the various types of fasciae (superficial, deep/muscular and visceral fasciae), while others have focused on their continuity, pointing out that their densities but not basic structures are variable. According to Guimberteau et al. [16], fascia is a structure that evolves hierarchically from a one cell embryo to the organism, and it is constantly adapting to new stresses to meet the organism’s structural demands. A similar description was given by Levin and Martin [17] who considered a fascia to be a tension network, with all the collagen inherently stressed—the so-called “pre-stress” of biologic tissues.
2. Classification of the Fasciae
At the Fourth International Fascia Research congress, which was held near Washington, DC in September of 2015, two definitions of fascia were proposed: it was, in fact, called both “a fascia” and “the fascial system” [18]. A fascia is “a sheath, a sheet, or any other dissectible aggregations of connective tissue that forms beneath the skin to attach, enclose, and separate muscles and other internal organs”. The fascial system “consists of the three-dimensional continuum of soft, collagen-containing, loose and dense fibrous connective tissues that permeate the body, providing an environment that enables all body systems to operate in an integrated manner”. The first is clearly a morphological/anatomical definition, while the second is a functional one. As the current study intends to examine the fascia primarily from an anatomical viewpoint, we will dedicate ourselves to exploring the first definition.
The fasciae can be distinguished into three main types depending on their histological characteristics and anatomical relationships:
- -Superficial fascia, which is a fibro-fatty layer in the middle of the subcutaneous adipose tissue, dividing it in a superficial part (SAT: superficial adipose tissue) and in a deep one (DAT: deep adipose tissue). The superficial fascia can show stratification both grossly and microscopically. It is conventionally described as being made up of membranous layers with loosely packed interwoven collagen and elastic fibers more superficial than other types and containing more elastic fibers [19];
- -Deep/muscular fasciae: depending on their orientations, composition architectures, and anatomical locations, the two main types of deep/muscular fasciae are the aponeurotic fasciae and the epimysial fasciae [13][20][13,20]. The former refers to all the “well-defined fibrous sheaths that cover and keep in place a group of muscles or serve for the insertion of a broad muscle”, as in the case of the deep fasciae of the limbs, the thoracolumbar fascia and the rectus sheath of the abdomen [21]. It works like a bridge connecting different muscles involved in a specific movement. The latter refers to the connective tissue sheath surrounding each skeletal muscle and penetrating them (as perimisium and endomisium). The epimysial fasciae define the shape of the muscles and play a key role in the peripheral motor coordination thanks to their strong relationship with the muscle spindles and organ corpuscles.
- -Visceral fasciae, which are distinguished in insertional and investing ones, according with their macroscopic and microscopic aspects. The investing fasciae are closely connected to individual organs and giving shape to them, support the parenchyma. The insertional fasciae are fibrous sheets forming the compartments for the organs and connect them to the musculoskeletal system [22];
- -Neural fasciae, which are all the meningeal layers and the connective tissues that envelop the peripheral nerves.
The deep/muscular fasciae have been receiving a great deal of attention over recent years in view of their possible role in proprioception, motor coordination and pain. Given the relevance of the[1][2][3][4][5][6][7][8]se three elements, the current study will focus on the microscopic characteristics of the muscular fasciae.
3. Thickness of the Deep/Muscular Fasciae
Morphometrically, the aponeurotic fasciae of the limbs present a mean thickness of 1 mm (590–1453 μm). The fasciae are thicker in the inferior limbs and posteriorly. The deep fascia of the thigh is thinner in the proximal region and thicker near the knee (mean thickness = 926 μm, SD ± 156 μm). In the lateral region, it is reinforced by the iliotibial tract. In the superior limbs, the brachial fascia presents a mean thickness of 863 μm (SD ± 77 μm); it is thinner in the anterior region and thicker in the posterior one. The antebrachial fascia has a mean thickness of 755 μm (SD ± 115 μm) and presents two reinforcements: the lacertus fibrosus and the retinacula of the wrist. These dimensions are consistent with those reported by Pirri et al. [23], whose in vivo data were obtained via ultrasound evaluation. This study, which evaluated the deep fasciae of different regions of the thigh, uncovered a mean thickness of 556.8 ± 176.2 µm in the anterior compartment, of 820.4 ± 201 µm in the medial one, of 1112 ± 237.9 µm in the lateral one, and of 730.4 ± 186.5 µm in the posterior one. When Wilke et al. [24] performed an ultrasound evaluation of the various fasciae in younger and older age groups, they found that young adults exhibited higher fascia thickness of the anterior and posterior lower leg, anterior thigh and abdominal wall (+12.3–25.8%, p < 0.05). The older groups instead showed higher thickness in the thoracolumbar fascia (2.35 versus 1.33 mm, +40.0–76.7%, p < 0.05). Correlations have been found between body mass and fascia thickness (τ = 0.45–0.75, p < 0.05) as well as between flexibility and fascia thickness (τ = 0.38–0.42, p < 0.05). We reported in one of our previous works that the thickness of the rectus sheath was significantly thicker in women who had undergone a caesarean section with respect to those who had undergone a vaginal delivery and/or healthy nulliparous women [25].
Reporting on the pectoral fascia of the epimysial fasciae, Stecco et al. [26] described a mean thickness of 150–200 µm. These investigators also noted that the fasciae were increasingly thicker towards the cranio-caudal direction. In fact, there was a mean thickness of 131 mm in the subclavicular region, 182 mm in the mammary region and 578 mm in the inferior thorax one. Wilke et al.’s [24] in vivo study evaluating the epimysial fascia of the erector spinae muscles found a mean thickness of 0.62 mm (0.4–1.75 mm).
Ultimately, the thickness of the muscular fascia seems to vary depending on its anatomical location, the individual’s body mass index (BMI), age and physiopathologic condition.
4. Microscopic Anatomy of the Deep/Muscular Fasciae
Many fibrous bundles running in different directions are macroscopically visible in the aponeurotic fasciae. This is why the aponeurotic fasciae were initially classified as irregular dense connective tissues. However, recent works [26][27][28][26,27,28] have demonstrated that the aponeurotic fasciae consist of two or three layers of parallel collagen fiber bundles, with each layer having a mean thickness of 277 µm (±SD 86.1 µm). The collagen fibers of adjacent layers may have different orientations. Each layer is separated from the adjacent one by a thin layer of loose connective tissue that permits them to slide over one another. Two thin layers (mean thickness 23 μm) of fibroelastic tissue are present on the external and internal sides of all the fasciae [26] (Figure 1).
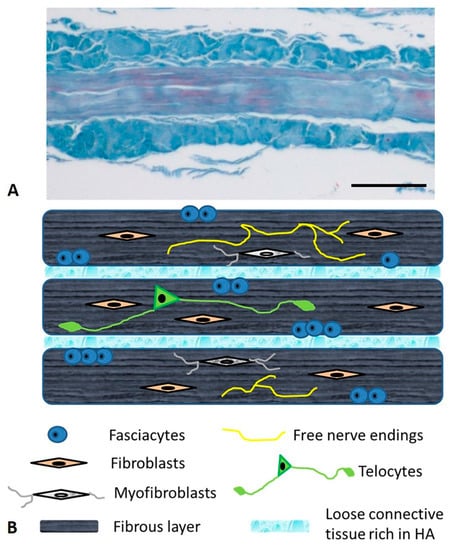
Figure 1. Microscopic anatomy of the human deep/muscular fascia. (A): Human fascia lata of the thigh—Azan Mallory staining Bar 250 µm; (B): schematic representation.
The epimysial fasciae are formed by undulated collagen fibers, arranged more or less transversely with respect to the underlying muscle, and they present an elevated number of elastic fibers which form an irregular mesh. The fascial layers providing intermuscular connections are able to transmit muscular force between adjacent synergistic muscles. The estimated percentage of elastic, with respect to collagen fibers, is approximately 15% [26]. Purslow et al. demonstrated that the epimysium has the same organization as the aponeurotic fasciae which consist of two or three sublayers of connective tissue and they described three sublayers of the epimysium: the internal, the middle and the external sublayers [29][30][29,30].
From a microanatomical point of view, the deep fascia is composed of various kinds of cells embedded in the extracellular matrix and there is an abundance of nervous fibers [31]. While the nervous components define the sensitive role of the fascial tissue, the cellular ones adapt the fascia to varying conditions, defining its metabolic properties and synthesizing the extracellular matrix. The latter is made up of two components: protein fibers and a water component. The former is essentially collagen type I, with some fibers of collagen type III and elastic fibers, which give support and organization to the structure. The latter, which is the ground substance, is a water-rich gelatinous substance containing abundant glycosaminoglycans, which confers turgor and flexibility to the tissue, permits the gliding property and transports metabolic material (Figure 1).
5. The Cells of the Fascial Tissue
The predominant cell population of the fascial tissue are fibroblasts [32][33][32,33]; their principal role is to maintain the structural integrity and organization of the tissue as they are involved in mechanotransduction and in secreting precursors of the extracellular matrix. As opposed to epithelial tissue, they are distributed randomly and not organized in flat monolayers or restricted to one side of the tissue.
The fasciacytes, small clusters of rounded cells along the surface of each fascial layer in the fascia lata, are specialized to the synthesis of hyaluronan (HA) of the ground substance [34]. They are fibroblast-like cells that are positive for the fibroblast marker vimentin, and their negativity to anti-CD68 has proven that they are not derived from the monocyte/macrophage lineage [35], as is the case for other HA-secreting cells, such as synoviocyte type B cells [36] or the hyalocytes of the eye [37][38][37,38]. The S100-A4-protein positive cells are markers that permit us to differentiate the cells from the classical fascial fibroblasts and to make the connection with the chondrocytes. They constitute markers for chondroid metaplasia or rather the reversible transformation towards a chondroid-like cell, despite the negative reaction to the marker of the chondrocyte family, collagen II [39]. In conclusion, it is a new type of cell, with a specialized function of HA synthesis and a rounded morphology; its cytoplasm is restricted to the perinuclear region, and it has smaller, less elongated cellular processes (Figure 2).
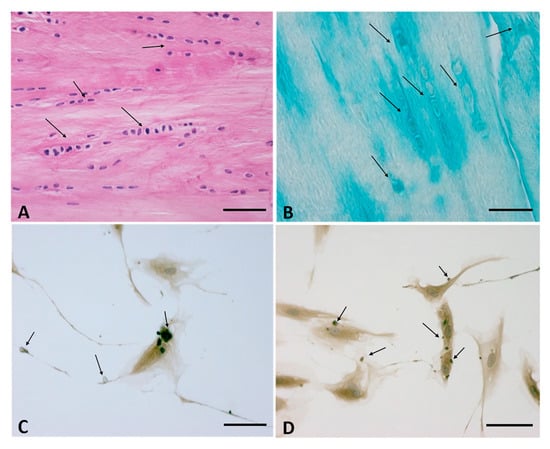
Figure 2. (A,B): Fasciacytes in Human fascia. (A): Hematoxylin and Eosin staining, Bar 50 μm—the nuclei were counterstained with hematoxylin; the Fasciacytes are highlighted by black arrows. (B): Alcian Blue/MgCl2 0.05 M, Bar 25 μm, the Fasciacytes are highlighted by black arrows. The fasciacytes have round nuclei, cytoplasm restricted to the perinuclear region, and smaller and less elongated cellular processes. (C,D): Human Monolayer Fascial fibroblasts treated with HU-308: Immunostaining with HABP Antibody. Arrows indicate HA positive vesicles. Bar 50 µm.
In normal healthy fascia, about 30% of the fibroblasts are fasciacytes, although this percentage may vary, depending on the stimuli to which the fascial tissue is subjected. The number and the specific localization of these cells in the sublayers of the fascial tissue is strictly correlated to their function: the fibroblasts produce the fibrous component (collagen and elastic fibers) and play a role in regulating force transmission at a distance. The fasciacytes instead produce hyaluronan, which allows fascial gliding between adjacent fascial sublayers.
The fasciae also contain myofibroblast cells which are specialized fibroblasts exhibiting contractile activities that regulate the basal tone of the tissue. The cell density of myofibroblasts in human fascia is different between body sites [40]. “In the human lumbar fascia [median 1.52% (IQR 0.17–4.89%), n = 12], it was found to be considerably higher than in the human plantar fascia and the fascia lata [0% (0–0%, n = 11, p = 0.003) versus 0% (0–0.03%, n = 12, p = 0.003)]” [40].The observed density of MFBs in human lumbar fascia in this study could possibly be associated with an augmented occurrence of (micro)injuries and related cellular repair processes in human lumbar fasciae. The contractile activity takes place via the adherens junctions, which open mechanosensitive ion channels in adjacent cells, resulting in Ca2+ influx [41]. The latter induces a contraction that can feed back on the first myofibroblast and/or stimulate other contacting myofibroblasts [41]. This coordination would gain importance during tissue remodeling when a high number of myofibroblasts contract the extracellular matrix (ECM) at the same time. The myofibroblasts are known to play a role in some pathological fibrotic contractures that affect the fascia. They may present abnormal proliferation, leading to a pathological contracture called Dupuytren disease that affects the palmar and digital fascia of the hand [42]. Some studies have recently outlined their role in normal fascial tissues; when viewed during a time-window of several minutes and longer, cellular contractions can impact motoneuronal coordination, musculoskeletal dynamics and regulation of fascial stiffness, inducing substantial contractures (~1 cm per month) [40][43][40,43].
Recent studies have also brought to light fascial structures containing telocytes, specialized connective tissue cells possessing long thin extensions called telopodes (Figure 3). Dawidowicz et al., who first described the presence of telocytes in the fascia lata [44][45][44,45], called them a “network in network” system, in view of the complex three-dimensional communication system that they form in the interstitial extracellular matrix. Telocytes have recently been identified in the tensor fascia lata, the crural fascia of the leg, the plantar fascia and the thoracolumbar fascia [46]. Although their specific role and distribution is still under investigation, these cells are believed to be involved in cell repair, regeneration, remodeling, immune control and cell communication via cell junctions or extracellular vesicles [47]; as such they probably play an important role in regulating myofascial pain and fascial disorders.

Figure 3. Telocyte in Human fascia-Semithin sections, Toluidine blue staining. Bar 25 µm. The telocytes are highlighted by black arrows.
References
- Pirri, C.; Todros, S.; Fede, C.; Pianigiani, S.; Fan, C.; Foti, C.; Stecco, C.; Pavan, P. Inter-rater reliability and variability of ultrasound measurements of abdominal muscles and fasciae thickness. Clin. Anat. 2019, 32, 948–960.Caterina Fede; Carmelo Pirri; Chenglei Fan; Lucia Petrelli; Diego Guidolin; Raffaele De Caro; Carla Stecco; A Closer Look at the Cellular and Molecular Components of the Deep/Muscular Fasciae. International Journal of Molecular Sciences 2021, 22, 1411, 10.3390/ijms22031411.
- Pirri, C.; Stecco, C.; Fede, C.; Macchi, V.; Özçakar, L. Ultrasound Imaging of the Fascial Layers: You See (Only) What You Know. J. Ultrasound Med. 2020, 39, 827–828.Nick D. Black; Carla Stecco; Vincent W. S. Chan; Fascial Plane Blocks: More Questions Than Answers?. Anesthesia & Analgesia 2020, 132, 899-905, 10.1213/ane.0000000000005321.
- Pirri, C.; Stecco, A.; Fede, C.; De Caro, R.; Stecco, C.; Özçakar, L. Ultrasound imaging of a scar on the knee: Sonopalpation for fascia and subcutaneous tissues. Eur. J. Transl. Myol. 2020, 30, 8909.Carmelo Pirri; Caterina Fede; Lucia Petrelli; Diego Guidolin; Chenglei Fan; Raffaele De Caro; Carla Stecco; An anatomical comparison of the fasciae of the thigh: A macroscopic, microscopic and ultrasound imaging study. Journal of Anatomy 2020, 238, 999-1009, 10.1111/joa.13360.
- Fan, C.; Fede, C.; Pirri, C.; Guidolin, D.; Biz, C.; Macchi, V.; De Caro, R.; Stecco, C. Quantitative Evaluation of the Echo Intensity of Paraneural Area and Myofascial Structure around Median Nerve in Carpal Tunnel Syndrome. Diagnostics 2020, 10, 914.Chenglei Fan; Caterina Fede; Carmelo Pirri; Diego Guidolin; Carlo Biz; Veronica Macchi; Raffaele De Caro; Carla Stecco; Quantitative Evaluation of the Echo Intensity of Paraneural Area and Myofascial Structure around Median Nerve in Carpal Tunnel Syndrome. Diagnostics 2020, 10, 914, 10.3390/diagnostics10110914.
- Almazán-Polo, J.; López-López, D.; Romero-Morales, C.; Rodríguez-Sanz, D.; Becerro-de-Bengoa-Vallejo, R.; Losa-Iglesias, M.E.; Bravo-Aguilar, M.; Calvo-Lobo, C. Quantitative Ultrasound Imaging Differences in Multifidus and Thoracolumbar Fasciae between Athletes with and without Chronic Lumbopelvic Pain: A Case-Control Study. J. Clin. Med. 2020, 9, 2647.Piero Pavan; Elena Monti; Michela Bondí; Chenglei Fan; Carla Stecco; Marco Narici; Carlo Reggiani; Lorenzo Marcucci; Alterations of Extracellular Matrix Mechanical Properties Contribute to Age-Related Functional Impairment of Human Skeletal Muscles. International Journal of Molecular Sciences 2020, 21, 3992, 10.3390/ijms21113992.
- Menon, R.G.; Oswald, S.F.; Raghavan, P.; Regatte, R.R.; Stecco, A. T1ρ-Mapping for Musculoskeletal Pain Diagnosis: Case Series of Variation of Water Bound Glycosaminoglycans Quantification before and after Fascial Manipulation® in Subjects with Elbow Pain. Int. J. Environ. Res. Public Health 2020, 17, 708.Caterina Fede; Carmelo Pirri; Lucia Petrelli; Diego Guidolin; Chenglei Fan; Raffaele De Caro; Carla Stecco; Sensitivity of the Fasciae to the Endocannabinoid System: Production of Hyaluronan-Rich Vesicles and Potential Peripheral Effects of Cannabinoids in Fascial Tissue. International Journal of Molecular Sciences 2020, 21, 2936, 10.3390/ijms21082936.
- Geneser, F. Textbook of Histology; Munksgaard: Copenhagen, Denmark, 1986.Caterina Fede; Andrea Porzionato; Lucia Petrelli; Chenglei Fan; Carmelo Pirri; Carlo Biz; Raffaele De Caro; Carla Stecco; Fascia and soft tissues innervation in the human hip and their possible role in post‐surgical pain. Journal of Orthopaedic Research 2020, 38, 1646-1654, 10.1002/jor.24665.
- Gray, H.; Standring, S.; Ellis, H.; Berkovitz, B.K.B. Gray’s Anatomy: The Anatomical Basis of Clinical Practice, 39th ed.; Elsevier Churchill Livingstone: New York, NY, USA, 2005.Caterina Fede; Carmelo Pirri; Chenglei Fan; Giovanna Albertin; Andrea Porzionato; Veronica Macchi; Raffaele De Caro; Carla Stecco; Sensitivity of the fasciae to sex hormone levels: Modulation of collagen-I, collagen-III and fibrillin production. PLoS ONE 2019, 14, e0223195, 10.1371/journal.pone.0223195.
- Bogduk, N.; Macintosh, J.E. The applied anatomy of the thoracolumbar fascia. Spine 1984, 9, 164–170.
- Martini, F.H.; Timmons, M.J.; Tallitsch, R.B. Anatomia Umana, 2nd ed.; EdiSES: Naples, Italy, 2004.
- Fawcett, D.W. Bloom & Fawcett: A Textbook of Histology, 12th ed.; McGraw-Hill: Milano, Italy, 1996.
- Gerlach, U.J.; Lierse, W. Functional construction of the superficial and deep fascia system of the lower limb in man. Acta Anat. 1990, 139, 11–25.
- Stecco, C. Functional Atlas of the Human Fascial System, 1st ed.; Elsevier: Edinburgh, UK, 2015; p. 4.
- Mense, S. Innervation of the thoracolumbar fascia. Eur. J. Transl. Myol. 2019, 29, 8297.
- Fede, C.; Porzionato, A.; Petrelli, L.; Fan, C.; Pirri, C.; Biz, C.; De Caro, R.; Stecco, C. Fascia and soft tissues innervation in the human hip and their possible role in post-surgical pain. J. Orthop. Res. 2020, 38, 1646–1654.
- Guimberteau, J.C.; Armstrong, C.; Findley, T.W.; Kapandji, M.D.; Adalbert, I. Architecture of Human Living Fascia: The Extracellular Matrix and Cells Revealed Through Endoscopy; Handspring Publishing: Pencaitland, East Lothian, Scotland, 2015.
- Levin, S.M.; Martin, D.C. Biotensegrity: The mechanics of fascia. In Fascia and the Tensional Network of the Human Body. The Science and Clinical Applications in Manual and Movement Therapy; Chapter: 3.5; Elsevier: Edinburgh, UK, 2012; pp. 137–142.
- Adstrum, S.; Hedley, G.; Schleip, R.; Stecco, C.; Yucesoy, C.A. Defining the fascial system. J. Bodyw. Mov. Ther. 2017, 21, 173–177.
- Gatt, A.; Agarwal, S.; Zito, P.M. Anatomy, Fascia Layers. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2020.
- Stecco, A.; Stern, R.; Fantoni, I.; De Caro, R.; Stecco, C. Fascial Disorders: Implications for Treatment. PM&R 2016, 8, 161–168.
- Stedman’s Medical Dictionary, 26th ed.; Williams & Wilkins: Baltimore, MD, USA, 1995; p. 628.
- Stecco, C.; Sfriso, M.M.; Porzionato, A.; Rambaldo, A.; Albertin, G.; Macchi, V.; De Caro, R. Microscopic anatomy of the visceral fasciae. J. Anat. 2017, 231, 121–128.
- Pirri, C.; Fede, C.; Petrelli, L.; Guidolin, D.; Fan, C.; De Caro, R.; Stecco, C. An anatomical comparison of the fasciae of the thigh: A macroscopic, microscopic and ultrasound imaging study. J. Anat. 2020.
- Wilke, J.; Macchi, V.; De Caro, R.; Stecco, C. Fascia thickness, aging and flexibility: Is there an association? J. Anat. 2019, 234, 43–49.
- Fan, C.; Guidolin, D.; Ragazzo, S.; Fede, C.; Pirri, C.; Gaudreault, N.; Porzionato, A.; Macchi, V.; De Caro, R.; Stecco, C. Effects of Cesarean Section and Vaginal Delivery on Abdominal Muscles and Fasciae. Medicina 2020, 56, 260.
- Stecco, A.; Macchi, V.; Masiero, S.; Porzionato, A.; Tiengo, C.; Stecco, C.; Delmas, V.; De Caro, R. Pectoral and femoral fasciae: Common aspects and regional specializations. Surg. Radiol. Anat. 2009, 31, 35–42.
- Benetazzo, L.; Bizzego, A.; De Caro, R.; Frigo, G.; Guidolin, D.; Stecco, C. 3D reconstruction of the crural and thoracolumbar fasciae. Surg. Radiol. Anat. 2011, 33, 855–862.
- Tesarz, J.; Hoheisel, U.; Wiedenhöfer, B.; Mense, S. Sensory innervation of the thoracolumbar fascia in rats and humans. Neuroscience 2011, 194, 302–308.
- Purslow, P.P. The structure and functional significance of variations in the connective tissue within muscle. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 133, 947–966.
- Purslow, P.P. The Structure and Role of Intramuscular Connective Tissue in Muscle Function. Front. Physiol. 2020, 11, 495.
- Kumka, M.; Bonar, J. Fascia: A morphological description and classification system based on a literature review. J. Can. Chiropr. Assoc. 2012, 56, 179–191.
- Langevin, H.M.; Cornbrooks, C.J.; Taatjes, D.J. Fibroblasts form a body-wide cellular network. Histochem. Cell Biol. 2004, 122, 7–15.
- Benjamin, M. The fascia of the limbs and back—A review. J. Anat. 2009, 214, 1–18.
- Stecco, C.; Fede, C.; Macchi, V.; Petrelli, L.; Biz, C.; Stern, R.; De Caro, R. The fasciacytes: A new cell devoted to fascial gliding regulation. Clin. Anat. 2018, 31, 667–676.
- Bartok, B.; Firestein, G.S. Fibroblast-like synoviocytes: Key effector cells in rheumatoid arthritis. Immunol. Rev. 2010, 233, 233–255.
- Iwanaga, T.; Shikichi, M.; Kitamura, H.; Yanase, H.; Nozawa-Inoue, K. Morphology and functional roles of synoviocytes in the joint. Arch. Histol. Cytol. 2000, 63, 17–31.
- Sakamoto, T.; Ishibashi, T. Hyalocytes: Essential cells of the vitreous cavity in vitreoretinal pathophysiology? Retina 2011, 31, 222–228.
- Holness, C.L.; Simmons, D.L. Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood 1993, 81, 1607–1613.
- Klein, D.M.; Katzman, B.M.; Mesa, J.A.; Lipton, J.F.; Caligiuri, D.A. Histology of the extensor retinaculum of the wrist and the ankle. J. Hand Surg. Am. 1999, 24, 799–802.
- Schleip, R.; Gabbiani, G.; Wilke, J.; Naylor, I.; Hinz, B.; Zorn, A.; Jäger, H.; Breul, R.; Schreiner, S.; Klingler, W. Fascia Is Able to Actively Contract and May Thereby Influence Musculoskeletal Dynamics: A Histochemical and Mechanographic Investigation. Front. Physiol. 2019, 10, 336.
- Follonier, L.; Schaub, S.; Meister, J.J.; Hinz, B. Myofibroblast communication is controlled by intercellular mechanical coupling. J. Cell Sci. 2008, 121, 3305–3316.
- Zhang, A.Y.; Kargel, J.S. The Basic Science of Dupuytren Disease. Hand Clin. 2018, 34, 301–305.
- Schleip, R.; Klingler, W. Active contractile properties of fascia. Clin. Anat. 2019, 32, 891–895.
- Dawidowicz, J.; Szotek, S.; Matysiak, N.; Mielańczyk, Ł.; Maksymowicz, K. Electron microscopy of human fascia lata: Focus on telocytes. J. Cell Mol. Med. 2015, 19, 2500–2506.
- Dawidowicz, J.; Matysiak, N.; Szotek, S.; Maksymowicz, K. Telocytes of Fascial Structures. Adv. Exp. Med. Biol. 2016, 913, 403–424.
- Blottner, D.; Huang, Y.; Trautmann, G.; Sun, L. The fascia: Continuum linking bone and myofascial bag for global and local body movement control on Earth and in Space. A scoping review. Reach 2019, 14–15, 100030.
- Chaitow, L. Telocytes: Connective tissue repair and communication cells. J. Bodyw. Mov. Ther. 2017, 21, 231–233.
