Inflammatory bowel disease (IBD) continue to cause substantial morbidity and massive productivity loss globally. IBD is more common among the productive generation (age group of <30yrs) and affects their quality of life. A single mechanism responsible for IBD is difficult to determine due to the complex interplay of multiple factors including host’s genetic predisposition and environmental factors. The relapsing nature of IBD demands repeated treatment implicating a substantial financial burden to individual patients and the concerned healthcare system, especially in developing nations. This review focuses on the causes of IBD,risk factors, current treatment options and challenges, the role played by the natural products in IBD health care; and situate these natural products within the current biodiscovery research agenda, including the applications of drug discovery techniques and the search for next generation drugs to treat IBD.
- IBD
- causes
- risk factors
- treatments
- drugs
- IBD, causes, risk factors, treatments, drugs
1. Introduction
The Hygiene Hypothesis is a central theme to the growing incidence of IBD, but it is still difficult to pinpoint which particular factors are responsible for causing IBD. Strachan first proposed the Hygiene Hypothesis in 1989 to explain the increasing incidence of atopy (allergic disorders)[1]. Later, many authors claimed through epidemiological studies and various experimental models that autoimmune disorders could be a result of broad environmental, infectious burden rather than individual behavior/hygiene[2][3][4][5]. According to the Rook’s reinterpretation of hygiene hypothesis (Old Friends Hypothesis as proposed in 2003), immunoregulatory disorders would occur first in those individuals with reduced/minimal contact with pathogens including commensal microbes and helminths (old friends) that are known to prime immunoregulation (Treg activity) in the human gut[6]. The rapid rise in the incidence of IBD over the last century in both developing and developed countries[7][8] could be related to improved hygiene practices such as access to clean water, non-contaminated food, and reduced family size[9]. While the definitive cause of IBD remains elusive and unknown, many studies point to 10 different causative factors (Figure 1)[10], with three major factors being genetic, environmental, and diet (which influences the host’s gut microbiota).
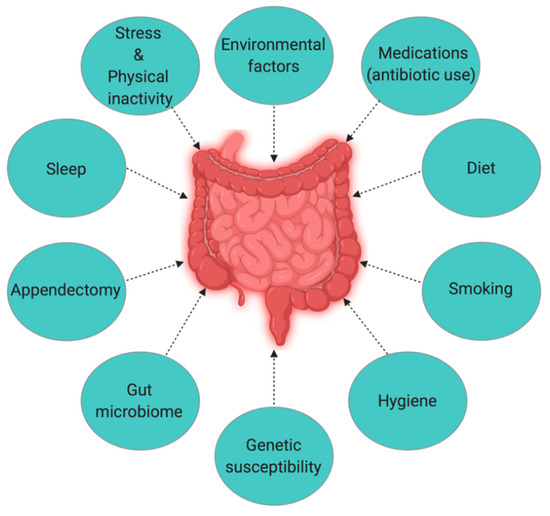
Figure 1. The interplay of factors causing inflammatory bowel disease (IBD).
2. Causes and Risk Factors
2.1. Genetics
Genetics
Genome-wide association studies identified 163 susceptible gene loci in IBD (with 110 common, 30 CD specific, and 23 UC specific)[11], highlighting the role of genetics in IBD pathogenesis. However, more than 50% of IBD susceptible gene loci are also associated with other inflammatory and autoimmune diseases[12]. MDR1 (multidrug resistance 1) gene in human chromosome 7 is one such gene that is associated with the pathogenesis of UC[13]. A comprehensive understanding of the mechanisms of IBD pathogenesis induced by genetic factors requires studying the role of an individual gene, which can be a challenging task. However, less than 50% concordance for IBD in twins represents the significant role that environmental factors might have in the development of IBD[14]. Another study conducted by Thompson et al. in British twins also reported similar (only 17% concordance for IBD) results among identical twins[15]. Moreover, the concordance between two disease conditions (CD and UC) among twins is not the same. Analysis of a Swedish twin cohort showed higher concordance (50%) for CD than for UC (18.8%), suggesting that genetic influence or heritability is higher in CD than in UC[14][16][17].
2.2. Environmental Factors
Environmental Factors
After extensive studies trying to determine the role of genetics in the pathogenesis of IBD have not yielded adequate evidence, many assume environmental factors to have a more significant role than genetics. Numerous studies have analyzed the linkage between various environmental factors and the development of IBD. When Khalili et al. studied the association of geographical variation with the incidence of IBD (both CD and UC) in two large prospective cohorts of US women (175,912), they found a higher incidence of both CD and UC among women residing in the northern latitudes, which could be attributable to less exposure to sunlight or ultraviolet B (UV-B) radiation[18]. UV-B radiation induces UV-Treg cells that have the potential to suppress inflammatory responses[19]. People living in higher altitudes are also more likely to have vitamin D deficiency due to insufficient exposure to sunlight[20]. Lack of vitamin D is also considered a possible cause of IBD since vitamin D receptor knock-out mice develop severe inflammation[21].
Numerous studies are suggesting a role for the environment in the pathogenesis of IBD, notably from a migration perspective[22][23][24]. For instance, children of immigrants who arrived in Canada at a younger age have an increased risk of IBD[25]. While Zoetendal et al. compared the risk of developing IBD among African-Americans and Africans inhabiting semi-urban (westernized) and rural environments respectively, the risk was significantly lower among those who were born and raised (first five years) in unhygienic environments like livestock farms compared to those living in the city[26]. Selective exposure to various environmental factors or conditions is also responsible for determining the microbiome composition. A study carried out in South Africa observed distinct gut microbiome composition in genetically similar populations in rural areas versus urban areas[27], where rural subjects contained significantly lesser Bacteriodetes populations than semi-urban and urban subjects. While a similar comparative study from India showed a dominance of phyla Bacteriodetes and Proteobacteria in the gut microbiome of rural subjects, phyla Firmicutes, and Lactobacillus in urban subject[28]. These findings indicate that exposure to different environmental conditions/lifestyles can influence the microbiome composition among a similar population.
2.3. Microbiota
Microbiota
The commensal human gut microbiota is essential for maintaining intestinal epithelial homeostasis and protection from mucosal injury[29]. The human lower GI tract has 1014 microbial cells[30], and Bacteriodetes and Firmicutes are the two most dominant phyla in the gut[31]. The gut microbiome determines the normal functioning of human health. For example, fibrinolytic bacteria degrade polysaccharides in the gut into smaller carbohydrates and short-chain fatty acids[32], and microbiota of the lower GI tract use dietary fiber as a source of energy[33]. Any changes in the composition of the healthy microbiota (dysbiosis) in the gut can trigger abnormal inflammatory responses. Firmicutes species, such as Faecalibacterium prausnitzii, has been reported to be poorly represented in patients with active IBD compared to healthy subjects[34]. The study conducted by Martin et al. further supports the protective role of this species, where intragastric administration of F. prausnitzii significantly decreased the severity of colitis in the trinitrobenzene sulfonic acid (TNBS)-induced mouse model of colitis[35]. The host must maintain a balance between recognizing pathogenic from commensal microbial species, as any disturbance in the composition of commensal species could trigger abnormal inflammatory responses such as IBD.
2.4. Diet and Smoking
Diet and Smoking
Diet influences the composition of the microbiota and their metabolic activity in the human gut[36]. There is a growing concern that the western diet, rich in fats and sugars, is responsible for the change in the diversity and metabolic activity of human gut microbiota, thereby contributing to the increasing incidence of IBD[37][38]. The increase in the abundance of Bilophila wadsworthia due to an animal-based diet can facilitate the growth of microorganisms that can trigger IBD[37][38]. Moreover, B. wadsworthia also produces hydrogen sulfide that can cause damage to intestinal tissues[36]. Long-term dietary pattern influences the development of IBD [39]. For instance, the intake of fruits decreases the risks of developing CD[40], although the underlying mechanism is yet to be understood. Smoking is one of the contradictory factors linked to IBD. While smoking is harmful to CD patients, reports show beneficial in UC[12]. The positive effect of smoking in UC is evident from the “Boston Drugs Surveillance Program”[41], ”UC patients in Birmingham, England”[42], and ”Oxford Family Planning Association Contraceptive Study”[43]. Additionally, the transdermal treatment of active UC patients with nicotine patches also showed better remission compared to the placebo group[44]. However, it is still controversial, and more research is required to determine if nicotine is one of the active components of cigarette smoking that is responsible for the beneficial effects on the UC disease course.
2.5. Sleep Deprivation, Stress, and Physical Inactivity
Sleep Deprivation, Stress, and Physical Inactivity
Inadequacy of sleep and psychological distress are additional intrinsic factors known to associated with inflammation and the inflammation system. Sleep disturbances are said to be common in IBD patients[45][46]. Some studies[47][48] have reported that symptoms of depression and anxiety cause clinical recurrence in IBD patients. However, stressful life events are not associated with the onset of inflammatory disease[49]. Alteration of sleep pattern or circadian rhythms[50] and insufficient sleep (<6 h/day)[46] has a direct impact on disease course and severity. A study involving 136 Japanese IBD patients found sleep disturbances as a potential risk factor of disease flare-up for both UC and CD within one year[45], but a similar kind of study (3173 IBD patients with sleep disturbances) conducted by Ananthakrishnan et al.[51] could observe an increased risk of disease flares only in CD within 6 months. A positive correlation between psychological distress and IBD flare-ups[52][53] indicates the need for timely psychological therapy in IBD patients.
2.6. Appendectomy
Appendectomy
Appendectomy (i.e., surgical removal of the appendix) and its association with the development of UC and CD is a scarcely explored area of research[54]. Few studies involving both humans as well as animal models showed evidence for a role of the appendix in gastroenterology. T-cell receptor-α mutant mice (TCR-α−/−) appendectomized at a young age (3–5 weeks old) contained more mesenteric lymph node (MLN) cells compared to the placebo group (sham-operated TCR- α−/− mice), indicating that the appendix could be an important site for priming MLN cells involved in causing IBD[55]. Similarly, Mombaerts et al. also found that an increase in the number of MLN cells in TCR-α−/− mice is related to the development of IBD[56]. A few studies and case reports have also shown the positive effect of appendectomy on the clinical course of UC in human subjects. A study of IBD patients in Australia confirmed that appendectomy before diagnosis delays disease onset of both UC and CD and results in fewer flare-ups in the case of UC when compared with patients without prior appendectomy[57]. A case report from Korea also confirmed that a patient with UC experienced a more extended period of remission after appendectomy[58]. However, the therapeutic relationship between CD and appendectomy remains inconclusive.[59].
2.7. Antibiotic Use
Antibiotic Use
A leading hypothesis in the etiology of IBD is the alteration in the human gut microbiota that triggers abnormal inflammatory responses, including IBD. Multiple factors are assumed to be responsible for inducing gut dysbiosis. Childhood exposure to antibiotics is one among them[60]. Children exposed to antibiotics at an early stage[60][61][62][63] and adults who had medication for acute gastroenteritis[64] possess higher risks for IBD. The frequency of use of antibiotics and the age at the time of use may have a varying effect as risks for IBD tend to decrease with increasing age at the time of exposure[65]. Regular intake of non-steroidal anti-inflammatory drugs like aspirin showed a strong positive correlation with only CD[66].
References
- D. P. Strachan; Hay fever, hygiene, and household size.. BMJ 1989, 299, 1259-1260, 10.1136/bmj.299.6710.1259.
- Jean-François Bach; The hygiene hypothesis in autoimmunity: the role of pathogens and commensals. Nature Reviews Immunology 2017, 18, 105-120, 10.1038/nri.2017.111.
- Greenwood, B.M.; Herrick, E.M.; Voller, A. Suppression of autoimmune disease in NZB and (NZB × NZW) F1 hybrid mice by infection with malaria. Nature 1970, 226, 266–267.
- Greenwood, B.M.; Herrick, E.M.; Voller, A. Can parasitic infection suppress autoimmune disease? Proc. R. Soc. Med. 1970, 63, 19–20.
- Diane L. Sewell; Emily Reinke; Laura H. Hogan; Matyas Sandor; Zsuzsanna Fabry; Immunoregulation of CNS autoimmunity by helminth and mycobacterial infections. Immunology Letters 2002, 82, 101-110, 10.1016/s0165-2478(02)00025-1.
- G.A.W. Rook; L R Brunet; Microbes, immunoregulation, and the gut. Gut 2005, 54, 317-320, 10.1136/gut.2004.053785.
- Sudabeh Alatab; Sadaf G Sepanlou; Kevin Ikuta; Homayoon Vahedi; Catherine Bisignano; Saeid Safiri; Anahita Sadeghi; Molly R Nixon; Amir Abdoli; Hassan Abolhassani; et al.Vahid AlipourMajid A H AlmadiAmir Almasi-HashianiAmir AnushiravaniJalal ArablooSuleman AtiqueAshish AwasthiAlaa BadawiAtif A A BaigNeeraj BhalaAli BijaniAntonio BiondiAntonio M BorzìKristin E BurkeFélix CarvalhoAhmad DaryaniManisha DubeyAziz EftekhariEduarda FernandesJoão C FernandesFlorian FischerArvin Haj-MirzaianArya Haj-MirzaianAmir HasanzadehMaryam HashemianSimon I HayChi L HoangMowafa HousehOlayinka S IlesanmiNader Jafari BalalamiSpencer L JamesAndre P KengneMasoud M MalekzadehShahin MeratTuomo J MeretojaTomislav MestrovicErkin M MirrakhimovHamid MirzaeiKarzan A MohammadAli H MokdadLorenzo MonastaIonut NegoiTrang H NguyenCuong T NguyenAkram PourshamsHossein PoustchiMohammad RabieeNavid RabieeKiana RamezanzadehDavid L RawafSalman RawafNima RezaeiStephen R RobinsonLuca RonfaniSonia SaxenaMasood SepehrimaneshMasood A ShaikhZeinab SharafiMehdi SharifSoraya SiabaniAli Reza SimaJasvinder A SinghAmin SoheiliRasoul SotoudehmaneshHafiz Ansar Rasul SuleriaBerhe E TesfayBach TranDerrick TsoiMarco VacanteAdam B WondmienehAfshin ZarghiZhi-Jiang ZhangMae DiracReza MalekzadehMohsen NaghaviGbd 2017 Inflammatory Bowel Disease Collaborators The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet Gastroenterology & Hepatology 2020, 5, 17-30, 10.1016/S2468-1253(19)30333-4.
- Gilaad G Kaplan; The global burden of IBD: from 2015 to 2025. Nature Reviews Gastroenterology & Hepatology 2015, 12, 720-727, 10.1038/nrgastro.2015.150.
- Mark A. Feeney; Frank Murphy; Andrew J. Clegg; Timothy M. Trebble; Nicholas M. Sharer; Jonathon A. Snook; A case–control study of childhood environmental risk factors for the development of inflammatory bowel disease. European Journal of Gastroenterology & Hepatology 2002, 14, 529-534, 10.1097/00042737-200205000-00010.
- Yi-Zhen Zhang; Yong-Yu Li; Inflammatory bowel disease: Pathogenesis. World Journal of Gastroenterology 2014, 20, 91-99, 10.3748/wjg.v20.i1.91.
- Luke Jostins; The International IBD Genetics Consortium (IIBDGC); Stephan Ripke; Rinse K. Weersma; Richard H. Duerr; Dermot P. McGovern; Ken Y. Hui; James C Lee; Phil Schumm; Yashoda Sharma; et al.Carl A. AndersonJonah EssersMitja MitrovicKaida NingIsabelle CleynenEmilie TheatreSarah SpainSoumya RaychaudhuriPhilippe GoyetteZhi WeiClara AbrahamJean-Paul AchkarTariq AhmadLeila AmininejadAshwin N. AnanthakrishnanVibeke AndersenJane M. AndrewsLeonard BaidooTobias BalschunPeter A. BamptonAlain BittonGabrielle BoucherStephan BrandCarsten BüningAriella CohainSven CichonMauro D'amatoDirk De JongKathy L. DevaneyMarla DubinskyCathryn EdwardsDavid EllinghausLynnette R. FergusonDenis FranchimontKarin FransénRichard B GearryMichel GeorgesChristian GiegerJürgen GlasTalin HarituniansAilsa HartChris HawkeyMatija HedlJ.P. HugotTom H. KarlsenLimas KupcinskasSubra KugathasanAnna LatianoDebby LaukensIan C. LawranceCharlie W. LeesEdouard LouisGillian MahyJohn MansfieldAngharad R. MorganCraig MowatWilliam G. NewmanOrazio PalmieriCyriel Y. PonsioenUroš PotočnikNatalie J. PrescottMiguel RegueiroJerome I. RotterRichard K. RussellJeremy D. SandersonMiquel SansJack SatsangiStefan SchreiberLisa A. SimmsJurgita SventoraityteStephan R. TarganKent D. TaylorMark TremellingHein W. VerspagetMartine De VosCisca WijmengaDavid C. WilsonJuliane WinkelmannRamnik J. XavierSebastian ZeissigBin ZhangClarence K. ZhangHongyu ZhaoMark S. SilverbergVito AnneseHakon HakonarsonSteven R. BrantGraham Radford-SmithChristopher G.P. MathewJohn D. RiouxEric E. SchadtMark J. DalyAndre FrankeMiles ParkesSéverine VermeireJeffrey C. BarrettJudy H ChoInternational IBD Genetics Consortium (IIBDGC) Host–microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012, 491, 119-124, 10.1038/nature11582.
- Khor, B.; Gardet, A.; Xavier, R.J.; Genetics and pathogenesis of inflammatory bowel disease.. Nature 2011, 474, 307–317, 10.1038/nature10209.
- R. Balfour Sartor; Mechanisms of Disease: pathogenesis of Crohn's disease and ulcerative colitis. Nature Clinical Practice Gastroenterology & Hepatology 2006, 3, 390-407, 10.1038/ncpgasthep0528.
- C Tysk; E Lindberg; G Jarnerot; B Flodérus-Myrhed; Ulcerative colitis and Crohn's disease in an unselected population of monozygotic and dizygotic twins. A study of heritability and the influence of smoking.. Gut 1988, 29, 990-996, 10.1136/gut.29.7.990.
- N. P. Thompson; R. Driscoll; R. E. Pounder; A. J. Wakefield; Genetics versus environment in inflammatory bowel disease: results of a British twin study.. BMJ 1996, 312, 95-96.
- Jonas Halfvarson; Lennart Bodin; Curt Tysk; Eva Lindberg; Gunnar Järnerot; Inflammatory bowel disease in a Swedish twin cohort: a long-term follow-up of concordance and clinical characteristics.. Gastroenterology 2003, 124, 1767-1773, 10.1016/s0016-5085(03)00385-8.
- L. Halme; Paulina Paavola-Sakki; Ulla Turunen; Maarit Lappalainen; Martti Farkkila; Kimmo Kontula; Family and twin studies in inflammatory bowel disease.. World Journal of Gastroenterology 2006, 12, 3668–3672.
- Hamed Khalili; Edward S Huang; Ashwin N Ananthakrishnan; Leslie Higuchi; James M Richter; Charles S Fuchs; Andrew T. Chan; Geographical variation and incidence of inflammatory bowel disease among US women. Gut 2012, 61, 1686-1692, 10.1136/gutjnl-2011-301574.
- Akira Maeda; Stefan Beissert; Thomas Schwarz; Agatha Schwarz; Phenotypic and functional characterization of ultraviolet radiation-induced regulatory T cells.. The Journal of Immunology 2008, 180, 3065-3071, 10.4049/jimmunol.180.5.3065.
- Sergio Setsuo Maeda; Gabriela Luporini Saraiva; Lilian Fukusima Hayashi; Maysa Seabra Cendoroglo; Luiz Roberto Ramos; Marcelo De Paula Corrêa; Carlos Henrique De Mesquita; Marise Lazaretti-Castro; Seasonal variation in the serum 25-hydroxyvitamin D levels of young and elderly active and inactive adults in São Paulo, Brazil: The São PAulo Vitamin D Evaluation Study (SPADES).. Dermato-Endocrinology 2013, 5, 211-7, 10.4161/derm.24476.
- Monica Froicu; Veronika Weaver; Thomas A. Wynn; Mary Ann McDowell; Jo Ellen Welsh; Margherita T. Cantorna; A Crucial Role for the Vitamin D Receptor in Experimental Inflammatory Bowel Diseases. Molecular Endocrinology 2003, 17, 2386-2392, 10.1210/me.2003-0281.
- Siew C Ng; Whitney Tang; Rupert W Leong; Minhu Chen; Yanna Ko; Corrie Studd; Ola Niewiadomski; Sally Bell; Michael A Kamm; H. J. De Silva; Anuradhani Kasturiratne; Yasith Udara Senanayake; Choon Jin Ooi; Khoon-Lin Ling; David Ong; Khean‐Lee Goh; Ida Hilmi; Qin Ouyang; Yu-Fang Wang; Pinjin Hu; Zhenhua Zhu; Zhirong Zeng; Kaichun Wu; Xin Wang; Bing Xia; Jin Li; Pises Pisespongsa; Sathaporn Manatsathit; Satimai Aniwan; Marcellus Simadibrata; Murdani Abdullah; Steve W C Tsang; Tai Chiu Wong; Aric J Hui; Chung Mo Chow; Hon Ho Yu; Mo Fong Li; Ka Kei Ng; Jessica Ching; Justin C Y Wu; Francis K L Chan; Joseph J.Y. Sung; Environmental risk factors in inflammatory bowel disease: a population-based case-control study in Asia-Pacific. Gut 2014, 64, 1063-1071, 10.1136/gutjnl-2014-307410.
- Vared Pinsk; Daniel Lemberg; Karan Grewal; Collin Barker; Richard A. Schreiber; Kevan Jacobson; Inflammatory Bowel Disease in the South Asian Pediatric Population of British Columbia. The American Journal of Gastroenterology 2007, 102, 1077-1083, 10.1111/j.1572-0241.2007.01124.x.
- C S Probert; V Jayanthi; D Pinder; A C Wicks; J F Mayberry; Epidemiological study of ulcerative proctocolitis in Indian migrants and the indigenous population of Leicestershire.. Gut 1992, 33, 687-693, 10.1136/gut.33.5.687.
- Eric I Benchimol; David R Mack; Astrid Guttmann; Geoffrey C Nguyen; Teresa To; Nassim Mojaverian; Pauline Quach; Douglas Manuel; Inflammatory Bowel Disease in Immigrants to Canada And Their Children: A Population-Based Cohort Study. The American Journal of Gastroenterology 2015, 110, 553-563, 10.1038/ajg.2015.52.
- Signe Timm; Cecilie Svanes; Christer Janson; Torben Sigsgaard; Ane Johannessen; Thorarinn Gislason; Rain Jõgi; Ernst Omenaas; Bertil Forsberg; Kjell Torén; et al.Mathias HolmLennart BråbäckVivi Schlünssen Place of upbringing in early childhood as related to inflammatory bowel diseases in adulthood: a population-based cohort study in Northern Europe.. European Journal of Epidemiology 2014, 29, 429-37, 10.1007/s10654-014-9922-3.
- Erwin G. Zoetendal; Philippe G. Puylaert; Junhai Ou; Kishore Vipperla; Faye M. Brouard; Elizabeth H. Ruder; Keith Newton; Franck Carbonero; H. Rex Gaskins; Willem M. De Vos; et al.Stephen J. O'keefe Sa1968 Distinct Microbiotas Are Present in Urban and Rural Native South Africans, and in African Americans. Gastroenterology 2013, 144, null, 10.1016/s0016-5085(13)61277-9.
- Bhabatosh Das; Tarini Shankar Ghosh; Saurabh Kedia; Ritika Rampal; Shruti Saxena; Satyabrata Bag; Ridhima Mitra; Mayanka Dayal; Ojasvi Mehta; A. Surendranath; et al.Simon P. L. TravisPrabhanshu TripathiG. Balakrish NairVineet Ahuja Analysis of the Gut Microbiome of Rural and Urban Healthy Indians Living in Sea Level and High Altitude Areas. Scientific Reports 2018, 8, 10104, 10.1038/s41598-018-28550-3.
- Seth Rakoff-Nahoum; Justin Paglino; Fatima Eslami-Varzaneh; Stephen Edberg; Ruslan Medzhitov; Recognition of Commensal Microflora by Toll-Like Receptors Is Required for Intestinal Homeostasis. Cell 2004, 118, 229-241, 10.1016/j.cell.2004.07.002.
- Nabeetha A. Nagalingam; S. V. Lynch; Role of the microbiota in inflammatory bowel diseases. Inflammatory Bowel Diseases 2012, 18, 968-984, 10.1002/ibd.21866.
- Paul B. Eckburg; Elisabeth M Bik; Charles N. Bernstein; Elizabeth Purdom; Les Dethlefsen; Michael Sargent; Steven R. Gill; Karen E. Nelson; David A Relman; Diversity of the Human Intestinal Microbial Flora. Science 2005, 308, 1635-1638, 10.1126/science.1110591.
- Xochitl C. Morgan; Timothy L Tickle; Harry Sokol; Dirk Gevers; Kathryn L Devaney; Doyle V Ward; Joshua A Reyes; Shiraz A. Shah; Neal Leleiko; Scott B Snapper; et al.Athos BousvarosJoshua KorzenikBruce E. SandsRamnik J. XavierCurtis Huttenhower Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biology 2012, 13, R79-R79, 10.1186/gb-2012-13-9-r79.
- H J Flint; Edward A. Bayer; Marco T. Rincon; Raphael Lamed; Bryan A. White; Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nature Reviews Microbiology 2008, 6, 121-131, 10.1038/nrmicro1817.
- Harry Sokol; Philippe Seksik; J. P. Furet; O. Firmesse; I. Nion-Larmurier; L. Beaugerie; J. Cosnes; G. Corthier; P. Marteau; J. Doré; et al. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflammatory Bowel Diseases 2009, 15, 1183-1189, 10.1002/ibd.20903.
- Rebeca Martín; Florian Chain; Sylvie Miquel; Jun Lu; Jean-Jacques Gratadoux; Harry Sokol; Elena F Verdu; Premysl Bercik; Luis G Bermúdez-Humarán; Philippe Langella; et al. The Commensal Bacterium Faecalibacterium prausnitzii Is Protective in DNBS-induced Chronic Moderate and Severe Colitis Models. Inflammatory Bowel Diseases 2014, 20, 417-430, 10.1097/01.mib.0000440815.76627.64.
- Lawrence A. David; Corinne F. Maurice; Rachel N. Carmody; David Gootenberg; Julie E. Button; Benjamin E. Wolfe; Alisha V. Ling; A. Sloan Devlin; Yug Varma; Michael A. Fischbach; et al.Sudha B. BiddingerRachel J. DuttonPeter J. Turnbaugh Diet rapidly and reproducibly alters the human gut microbiome. Nature 2013, 505, 559-563, 10.1038/nature12820.
- Suzanne Devkota; Yunwei Wang; Mark W. Musch; Vanessa Leone; Hannah Fehlner-Peach; Anuradha Nadimpalli; Dionysios A. Antonopoulos; Bana Jabri; Eugene B Chang; Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature 2012, 487, 104-8, 10.1038/nature11225.
- Peter J. Turnbaugh; Vanessa K. Ridaura; Jeremiah J. Faith; Federico E. Rey; Rob Knight; Jeffrey I. Gordon; The Effect of Diet on the Human Gut Microbiome: A Metagenomic Analysis in Humanized Gnotobiotic Mice. Science Translational Medicine 2009, 1, 6ra14-6ra14, 10.1126/scitranslmed.3000322.
- Gary D. Wu; Jun Chen; Christian Hoffmann; Kyle Bittinger; Ying-Yu Chen; Sue A. Keilbaugh; Meenakshi Bewtra; Dan Knights; William A. Walters; Rob Knight; et al.Rohini SinhaErin GilroyKernika GuptaRobert BaldassanoLisa NesselHongzhe LiFrederic D BushmanJames D. Lewis Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science 2011, 334, 105-108, 10.1126/science.1208344.
- Ashwin N. Ananthakrishnan; Hamed Khalili; Gauree G. Konijeti; Leslie M. Higuchi; Punyanganie De Silva; Joshua R. Korzenik; Charles S. Fuchs; Walter C. Willett; James M. Richter; Andrew T. Chan; et al. A prospective study of long-term intake of dietary fiber and risk of Crohn's disease and ulcerative colitis.. Gastroenterology 2013, 145, 970-7, 10.1053/j.gastro.2013.07.050.
- Hershel Jick; Alexander M. Walker; Cigarette Smoking and Ulcerative Colitis. New England Journal of Medicine 1983, 308, 261-263, 10.1056/nejm198302033080507.
- Gyde, S.; Prior, P.; Dew, M.J.; Saunders, V.; Waterhouse, J.A.; Allan, R.N.; Mortality in ulcerative colitis. Gastroenterology 1982, 83, 36–43.
- M Vessey; D Jewell; A Smith; D Yeates; K McPherson; Chronic inflammatory bowel disease, cigarette smoking, and use of oral contraceptives: findings in a large cohort study of women of childbearing age.. British medical journal (Clinical research ed.) 1986, 292, 1101-1103.
- Rupert D. Pullan; John Rhodes; Subramanian Ganesh; Venk Mani; John S. Morris; Geraint T. Williams; Robert G. Newcombe; Michael Russell; Colin Feyerabend; Gareth Thomas; et al.Urbain Säwe Transdermal Nicotine for Active Ulcerative Colitis. New England Journal of Medicine 1994, 330, 811-815, 10.1056/nejm199403243301202.
- Risa Uemura; Yasuhiro Fujiwara; Narika Iwakura; Masatsugu Shiba; Kenji Watanabe; Noriko Kamata; Hirokazu Yamagami; Tetsuya Tanigawa; Toshio Watanabe; Kazunari Tominaga; Tetsuo Arakawa; Sleep disturbances in Japanese patients with inflammatory bowel disease and their impact on disease flare.. SpringerPlus 2016, 5, 1792, 10.1186/s40064-016-3408-6.
- Ashwin N. Ananthakrishnan; Hamed Khalili; Gauree G. Konijeti; Leslie M. Higuchi; Punyanganie De Silva; Charles S. Fuchs; James M. Richter; Eva Schernhammer; Andrew T. Chan; Sleep duration affects risk for ulcerative colitis: a prospective cohort study.. Clinical Gastroenterology and Hepatology 2014, 12, 1879-86, 10.1016/j.cgh.2014.04.021.
- Antonina Mikocka‐Walus; Claudia Anderegg; Peter Bauerfeind; Christoph Beglinger; Stefan Begré; Dominique Belli; José M. Bengoa; Luc Biedermann; Beat Bigler; Janek Binek; Mirjam Blattmann; Stephan Boehm; Jan Borovicka; Christian P. Braegger; Nora Brunner; Patrick Bühr; Bernard Burnand; Emanuel Burri; Sophie Buyse; Matthias Cremer; Dominique H. Criblez; Philippe De Saussure; Lukas Degen; Joakim Delarive; Christopher Doerig; Barbara Dora; Gian Dorta; Mara Egger; Tobias Ehmann; Ali El-Wafa; Matthias Engelmann; Jessica Ezri; Christian Felley; Markus Fliegner; Nicolas Fournier; Montserrat Fraga; Pascal Frei; Remus Frei; Michael Fried; Florian Froehlich; Christian Funk; Raoul Ivano Furlano; Suzanne Gallot-Lavallée; Martin Geyer; Marc Girardin; Delphine Golay; Tanja Grandinetti; Beat Gysi; Horst Haack; Johannes Haarer; Beat Helbling; Peter Hengstler; Denise Herzog; Cyrill Hess; Klaas Heyland; Thomas Hinterleitner; Philippe Hiroz; Claudia Hirschi; Petr Hruz; Rika Iwata; Res Jost; Pascal Juillerat; Vera Kessler Brondolo; Christina Knellwolf; Christoph Knoblauch; Henrik Köhler; Rebekka Koller; Claudia Krieger-Grübel; Gerd Kullak-Ublick; Patrizia Künzler; Markus A. Landolt; Rupprecht Lange; Frank Serge Lehmann; Andrew MacPherson; Philippe Maerten; Michel H. Maillard; Christine Manser; Michael Manz; Urs Marbet; George Marx; Christoph Matter; Valérie McLin; Rémy Meier; Martina Mendanova; Christa Meyenberger; Pierre Michetti; Benjamin Misselwitz; Darius Moradpour; Bernhard Morell; Patrick Mosler; Christian Mottet; Christoph Müller; Pascal Müller; Beat Mullhaupt; Claudia Münger-Beyeler; Leilla Musso; Andreas Nagy; Michaela Neagu; Cristina Nichita; Jan Niess; Natacha Noël; Andreas Nydegger; Nicole Obialo; Carl Oneta; Cassandra Oropesa; Ueli Peter; Daniel Peternac; Laetitia Marie Petit; Franziska Piccoli-Gfeller; Julia Beatrice Pilz; Nadia Raschle; Ronald Rentsch; Sophie Restellini; Jean-Pierre Richterich; Sylvia Rihs; Marc Alain Ritz; Jocelyn Roduit; Daniela Rogler; Gerhard Rogler; Jean-Benoît Rossel; Markus Sagmeister; Gaby Saner; Bernhard Sauter; Mikael Sawatzki; Michela Schäppi; Michael Scharl; Martin Schelling; Susanne Schibli; Hugo Schlauri; Sybille Schmid Uebelhart; Jean-François Schnegg; Alain Schoepfer; Frank Seibold; Mariam Seirafi; Gian-Marco Semadeni; David Semela; Arne Senning; Marc Sidler; Christiane Sokollik; Johannes Spalinger; Holger Spangenberger; Philippe Stadler; Michael Steuerwald; Alex Straumann; Bigna Straumann-Funk; Michael Sulz; Joël Thorens; Sarah Tiedemann; Radu Tutuian; Stephan Vavricka; Francesco Viani; Jürg Vögtlin; Roland Von Känel; Alain Vonlaufen; Dominique Vouillamoz; Rachel Vulliamy; Jürg Wermuth; Helene Werner; Paul Wiesel; Reiner Wiest; Tina Wylie; Jonas Zeitz; Dorothee Zimmermann; Valerie Pittet; Valerie Pittet; Symptoms of Depression and Anxiety Are Independently Associated With Clinical Recurrence of Inflammatory Bowel Disease. Clinical Gastroenterology and Hepatology 2016, 14, 829-835.e1, 10.1016/j.cgh.2015.12.045.
- Antonina Mikocka‐Walus; Wayne Massuger; Simon R Knowles; Gregory T Moore; Stephanie Buckton; William Connell; Paul Pavli; Leanne Raven; Jane M Andrews; Psychological distress is highly prevalent in inflammatory bowel disease: A survey of psychological needs and attitudes. JGH Open 2020, 4, 166-171, 10.1002/jgh3.12236.
- Eric Lerebours; Corinne Gower; Veronique Merle; Franck Brazier; Stéphane Debeugny; Raymond Marti; Jean Louis Salomez; Marie France Hellot; Jean Louis Dupas; Jean–Frederic Colombel; et al.Antoine CortotJacques Benichou Stressful Life Events as a Risk Factor for Inflammatory Bowel Disease Onset: A Population-Based Case–Control Study. The American Journal of Gastroenterology 2007, 102, 122-131, 10.1111/j.1572-0241.2006.00931.x.
- Garth R Swanson; Helen J Burgess; Ali Keshavarzian; Sleep disturbances and inflammatory bowel disease: a potential trigger for disease flare?. Expert Review of Clinical Immunology 2011, 7, 29-36, 10.1586/eci.10.83.
- Ashwin N. Ananthakrishnan; Millie D. Long; Christopher F. Martin; Robert S. Sandler; Michael D. Kappelman; Sleep disturbance and risk of active disease in patients with Crohn's disease and ulcerative colitis.. Clinical Gastroenterology and Hepatology 2013, 11, 965-71, 10.1016/j.cgh.2013.01.021.
- H. E. Mardini; Kevin E. Ki; John W. Wilson; Crohn's Disease: A Two-Year Prospective Study of the Association Between Psychological Distress and Disease Activity. Digestive Diseases and Sciences 2004, 49, 492-497, 10.1023/b:ddas.0000020509.23162.cc.
- Susan Levenstein; Cosimo Prantera; Vilma Varvo; Maria Lia Scribano; Arnaldo Andreoli; Carlo Luzi; Massimo Arcà; Eva Berto; Giustina Milite; Adriana Marcheggiano; Stress and exacerbation in ulcerative colitis: a prospective study of patients enrolled in remission. The American Journal of Gastroenterology 2000, 95, 1213-1220, 10.1016/s0002-9270(00)00804-2.
- G. L. Radford-Smith; What is the importance of appendectomy in the natural history of IBD?. Inflammatory Bowel Diseases 2008, 14, S72-S74, 10.1002/ibd.20623.
- A. Mizoguchi; C Chiba; A K Bhan; Role of appendix in the development of inflammatory bowel disease in TCR-alpha mutant mice.. Journal of Experimental Medicine 1996, 184, 707-715, 10.1084/jem.184.2.707.
- Peter Mombaerts; Emiko Mizoguchi; Michael J. Grusby; Laurie H. Glimcher; Atul K. Bhan; Susumu Tonegawa; Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell 1993, 75, 275-282, 10.1016/0092-8674(93)80069-q.
- G L Radford-Smith; J E Edwards; D M Purdie; N. Pandeya; M Watson; Nicholas G. Martin; A Green; B Newman; Timothy Hj Florin; Protective role of appendicectomy on onset and severity of ulcerative colitis and Crohn's disease. Gut 2002, 51, 808-813, 10.1136/gut.51.6.808.
- Chee Ho Noh; Dae Young Cheung; Tae Ho Kim; Eun Jung Jun; In Kyu Lee; Jin Il Kim; Se Hyun Cho; Soo-Heon Park; Joon Yeol Han; Jae Kwang Kim; et al. [Remission of ulcerative colitis after appendectomy: a case report].. The Korean Journal of Gastroenterology 2010, 56, 201-204, 10.4166/kjg.2010.56.3.201.
- A Dignass; Rami Eliakim; Fernando Magro; Christian Maaser; Yehuda Chowers; Karel Geboes; Gerassimos Mantzaris; Walter Reinisch; Jean-Frederic Colombel; Séverine Vermeire; et al.Simon TravisJames O. LindsayGert Van Assche Second European evidence-based consensus on the diagnosis and management of ulcerative colitis Part 1: Definitions and diagnosis. Journal of Crohn’s and Colitis 2012, 6, 965-990, 10.1016/j.crohns.2012.09.003.
- Souradet Y Shaw; James F Blanchard; Charles N. Bernstein; Association Between the Use of Antibiotics in the First Year of Life and Pediatric Inflammatory Bowel Disease. The American Journal of Gastroenterology 2010, 105, 2687-2692, 10.1038/ajg.2010.398.
- Anders Hviid; H. Svanstrom; Morten Frisch; Antibiotic use and inflammatory bowel diseases in childhood. Gut 2010, 60, 49-54, 10.1136/gut.2010.219683.
- Souradet Y Shaw; James F Blanchard; Charles N. Bernstein; Association Between the Use of Antibiotics and New Diagnoses of Crohnʼs Disease and Ulcerative Colitis. The American Journal of Gastroenterology 2011, 106, 2133-2142, 10.1038/ajg.2011.304.
- Lauri Virta; Anssi Auvinen; Hans Helenius; Pentti Huovinen; Kaija‐Leena Kolho; Association of Repeated Exposure to Antibiotics With the Development of Pediatric Crohn's Disease--A Nationwide, Register-based Finnish Case-Control Study. American Journal of Epidemiology 2012, 175, 775-784, 10.1093/aje/kwr400.
- Garcia Rodriguez, L.A.; Ruigomez, A.; Panes, J.; Acute gastroenteritis is followed by an increased risk of inflammatory bowel disease. Yearbook of Medicine 2006, 130, 1588–1594.
- Kronman, M.P.; Zaoutis, T.E.; Haynes, K.; Feng, R.; Coffin, S.E.; Antibiotic Exposure and IBD Development Among Children: A Population-Based Cohort Study. Pediatrics 2012, 130, e794-e803, 10.1542/peds.2011-3886 .
- Simon S M Chan; Robert N. Luben; M. M. Bergmann; H. Boeing; Anja Olsen; Anne Tjønneland; Kim Overvad; Rudolf Kaaks; H. Kennedy; Kay-Tee Khaw; et al.Elio RiboliA. R. Hart Aspirin in the aetiology of Crohn’s disease and ulcerative colitis: a European prospective cohort study. Alimentary Pharmacology and Therapeutics 2011, 34, 649-655, 10.1111/j.1365-2036.2011.04784.x.
