Thymoquinone (TQ) is the most pharmacologically active ingredient in Nigella sativa seeds (black seeds); it is reported to have anticancer, anti-inflammatory and antioxidant effects in various settings.
- Thymoquinone
- COVID-19
1. Introduction
In March 2020, the WHO declared coronavirus disease -2019 (COVID-19) as a pandemic; this served as the spark that ignited the global race for the development of a vaccine against the novel coronavirus, severe acute respiratory syndrome-coronavirus-2(SARS-CoV-2). This was evidently important amidst the sudden and substantial increase in hospitalizations for pneumonia associated with multi-organ disease. The pathophysiology of COVID-19 patients gave clues to suitable supportive and symptomatic treatment of this pandemic; however, no specific treatment against COVID-19 has been found to date. COVID-19 patients have many symptoms that vary in severity, including dry cough, fever [1], sore throat, fatigue, diarrhea, shortness of breath, myalgia, in addition to radiographic and laboratory or biochemical abnormalities [2]. Moreover, cardiovascular manifestations include. Acute cardiac injury, myocarditis, arrhythmia and cardiovascular thromboembolism havebeen frequently reported in COVID-19 patients [3]. Furthermore, dizziness, headache, taste and smell dysfunctions, or impaired consciousness has been frequently shown among neurological manifestations in COVID-19 patients [4][5][4,5]. Cases involving either viral co-infection or co-infection with both viral and bacterial pathogens that cause pneumonia have been described, particularly in the period following the initial phase of viral respiratory infection [6]. In severe cases, acute lung injury (ALI), acute respiratory distress syndrome (ARDS), respiratory failure, heart failure, sepsis, multiple organ dysfunction, and sudden cardiac arrest occur within a few days [7]. The supportive treatments include antipyretics, antiviral therapies, different combinations of broad-spectrum antibiotics, hydroxychloroquine and plasma transfusion [8]. Cancer patients are at higher risk (two-folds) for COVID-19 and are more likely to develop severe outcomes compared to the general population [9][10][11][9,10,11]. This is greatly attributed to immunosuppressive conditions, consumption of multiple medications, combined comorbidity, and are more likely to require ventilatory support or ICU admission [12][13][14][15][12,13,14,15]. They also have significantly higher fatality rates (28.6% compared to 2.3% for all COVID-19 patients in China) [12]. Patients who have received cancer treatment within 14 days of getting a COVID-19 diagnosis are considered to have a risk factor for developing adverse events, such as ARDS, septic shock, and acute myocardial infarction [12]. Despite this observation, delaying cancer treatment for patients also has well-documented complications [12]. It may seem rather challenging to discover a drug that shows not only antiviral effects specifically against SARS-CoV-2 infection and entry but also antimicrobial (antiviral, -bacterial, -fungal) that protects against SARS-CoV-2 co-infection induced pneumonia, ameliorate respiratory symptoms, as well as acts as an antioxidant thereby protecting against COVID-19 associated multiple organ dysfunction (i.e., cardio-protective, hepato-protective, gastro-protective and protects the kidney from injury). Moreover, this drug should also ideally have anti-inflammatory effects that are sufficient enough to ameliorate COVID-19 induced cytokine storm and whose overall pharmacological effects are consistent with the pathophysiology of COVID-19 in cancer patients.
TQ shows all the above-mentioned effects [16][17][18][19][20][21][22][23][24][25][26][16,17,18,19,20,21,22,23,24,25,26]. TQ is the most pharmacologically active ingredient in Nigella sativa seeds (black seeds) extract [27][28][27,28]. In this review, we will be discussing the potential effects of TQ as a SARS-CoV-2 antiviral drug, its beneficial effects against COVID-19 pathophysiology with a focus on cancer patients, as well as some of its anticancer effects and its use as an adjuvant besides supportive COVID-19 therapy and cancer therapy. To achieve the purpose ofthe review, research was conducted at the States National Library of Medicine (PubMed). For the search in databases, the descriptors used were: “thymoquinone” and “COVID-19”/“SARS-CoV-2” or “cancer”, “COVID-19”/“SARS-CoV-2” and “cancer” or “apoptosis”, “thymoquinone” and various COVID-19 complications and key molecular pathways.
2. Thymoquinone’s Double Hits in COVID-19 Infected Cancer Patients
First of all, a recent study showed that TQ could work as an antiviral against SARS-CoV-2 since it revealed that TQ mighthave inhibitory activities against its viral protease in silico [29][161], and several other studies are also working on proving the same effect through insilico computational analysis. This rather interesting observation prompted the analysis of TQ’s reported roles in regulating proteins that are involved in COVID-19 as well as in cancer pathogenesis. This review, therefore, aims to summarize in the following sections the common protein targets that are subject to regulation by TQ and whose roles are furthermore crucial in COVID-19 cancer patients.
Heat shock protein A5 (HSPA5)/GRP78 is also known as immunoglobulin heavy chain-binding protein (BiP) or glucose-regulated protein (GRP78). GRP78 overexpression can occur under a variety of stressful conditions. This overexpression can cause the protein to be, in turn, highly abundant on the cell surface, where it can further potentiate the stressful conditions under which its expression was increased. For instance, overexpression of GRP78 on the cell membranes can increase viral entry via recognition and binding to the substrate-binding domain (SBD) of GRP78. This is true for several viruses [30][162], and molecular docking also showed that SARS-CoV-2 spike protein could bind to the host cell surface GRP78 [31][163]. Furthermore, the literature confirmed the in vitro presence of GRP78 protein in airway epithelial cells and in situ protein expression of GRP78 in the respiratory mucosa [32][164]. Higher serum GRP78 concentrations were found in COVID-19 patients compared to patients with pneumonia and the control group [33][165]. In another example, GRP78 can be overexpressed in the setting of a malignancy, where its abundance on cancer cell membranes endows antiapoptotic properties to the tumor cell by increasing the levels of antiapoptotic proteins such as Bcl-2 and reducing the levels of proapoptotic proteins, such as Bax. Ultimately, this results in promoting tumor survival, progression, angiogenesis, invasion, metastasis, as well as resistance to therapy [34][166]. This has been observed in various types of cancers [34][35][36][37][38][39][167,168,169,170,171].
HSPA5/GRP78 and TQ: TQ decreases the expression of HSPA5/GRP78 and improved mitochondrial function [40][172]. Furthermore, molecular docking showed that TQ might interfere with SARS-CoV-2 attachment to HSPA5 by tightly binding to this protein on the cell surface [41][173]. Collectively, TQ produces a dual hit, where it tightly binds to HSPA5/GRP78, reducing its expression. Thus both reduces the risk of SARS-CoV-2 infection and decrease chemotherapy resistance, cancer invasion, metastasis, and survival, as illustrated briefly in FigureFigure 2 2 [41][173].
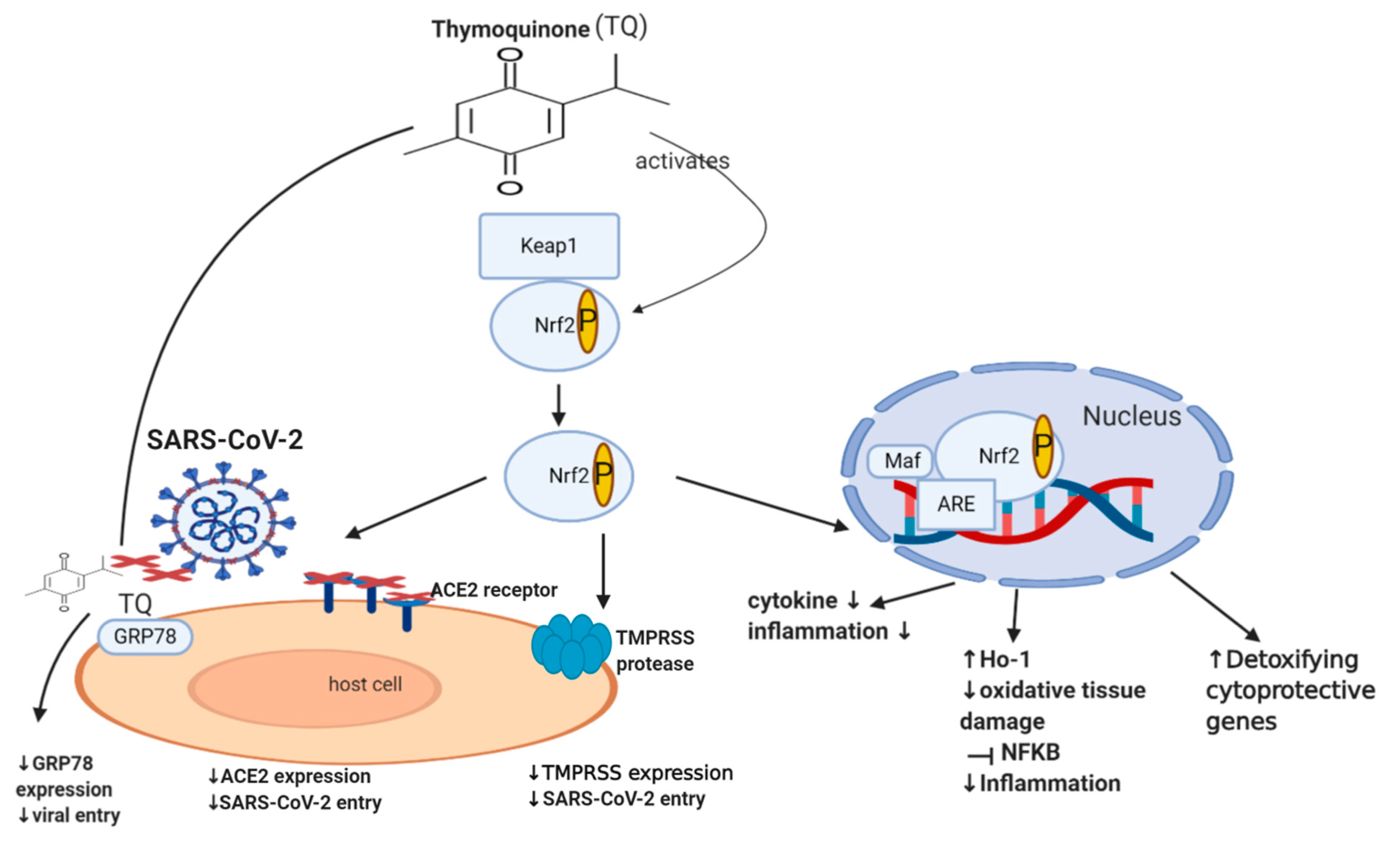
Nrf2 (nuclear factor erythroid-derived 2 related factor 2) is an important transcription factor that counteracts oxidative stress, where it acts as a sensor of oxidative stress, preventing genomic instability. It regulates about 250 genes involved in cellular homeostasis, including detoxifying enzymes, antioxidant proteins, and cytoprotective proteins [61][174]. Under normal physiological conditions, Nrf2 is sequestered by the cytoplasmic keap1 (Kelch-like ECH-associated protein 1), which maintains the Nrf2 at low levels through targeting it for proteasomal degradation (ubiquitination) [62][175]. Disruption of Nrf2 homeostasis can be seen in various settings, including viral infection and cancer. During a viral infection, intracellular expression of viral proteins leads to an increase in the oxidative stress of a cell. This leads to the dissociation of Nrf2 from keap1, which consequently prevents its ubiquitination [63][64][176,177]. Nrf2 is now able to translocate to the nucleus and activate the transcription of detoxifying, cytoprotective genes such as heme oxygenase (HO-1) [65][66][178,179]. This mechanism is also involved in the setting of cancer, where Nrf2 is additionally able to protect a cell from chemical and radiation-induced carcinogenesis [42][43][44][45][180,181,182,183]. Moreover, literature revealed that Nrf2 is able to enhance innate immune system activity, as well as participate in the inhibition of inflammatory cytokine expression, including IL-1β, IL-6, and NF-ĸB, ultimately decreasing inflammation [46][47][67][184,185,186]. Although some studies show that the continuous activation of Nrf2, as a result of excessive levels of ROS, could have deleterious effects on the host cell [68][69][187,188], it usually does not happen in viral infections since the virus needs to keep optimal oxidative stress levels allowing it to maintain viral metabolism without causing death in the host cell [70][189]. A recent study in 2020 involving 40 patients showed that the severity of COVID-19 is inversely associated with Nrf2 expression and directly linked to age and intensity of inflammatory response [71][190]. Interestingly, recent studies showed that Nrf2 deficiency upregulates ACE2 receptors, while activation of Nrf2 downregulates ACE2 receptors, with Nrf2 knock-out mice showing enhanced ACE2 expression. In cultured immortalized renal proximal tubule cells, treatment with Nrf2 inhibitor (Trigonelline) or transfection with Nrf2 small interfering RNA led to an increase in ACE2 transcription [48][191]. The exact mechanism of how Nrf2 downregulates the ACE2 receptor remains unclear. Additionally, Nrf2 activators downregulate the mRNA expression of TMPRSS2 [49][50][192,193] via upregulating TMPRSS2 inhibitors PAI-1 plasminogen activator inhibitor-1 encoded by the SERPINE gene (SERPINE/PAI-1) [49][192] and secretory leukocyte protease inhibitor (SLPI) [50][193]. This may highlight the important role of Nrf2 in downregulating TMPRSS2, ACE2 receptor expression and subsequently decreasing SARS-CoV-2 infection load. Despite the numerous cytoprotective mechanisms of Nrf2, it still appears to be a double-edged molecule since there is cumulative evidence establishing the fact that Nrf2 is one of the pathways that drive cancer progression, spread or metastasis, and chemo-resistance [51][52][53][54][55][56][57][58][59][60][194,195,196,197,198,199,200,201,202,203], and hence further dedicated studies may still be required.
The Renin-Angiotensin System (RAS) is a homeostatic loop that begins when the hepatic angiotensinogen is converted into angiotensin I (ATI) by the renal renin enzyme. This loop then involves two arms; the ACE enzyme (from the lungs) converts ATI to angiotensin II (ATII), increasing its circulating levels. This mediator is implicated in vasoconstriction, fibrosis, hypertension, and inflammation. The second arm involves the conversion of ATII to AT1-7 by ACE2, which carries out the opposite effects of its precursor, ATII [72][73][242,243]. Many of the cardiovascular symptoms, as well as multiple organ damage seen with COVID-19, can be linked to RAS-induced dysregulation [74][244]. In fact, patients with COVID-19 tend to have significantly higher levels of circulating ATII compared to healthy individuals, and the findings of various studies that were conducted on autopsies [75][245] suggest that SARS-CoV-2 may cause downregulation of ACE2 receptors. This finding, along with receptor internalization upon viral binding, further contribute to the increase in ATII levels and the imbalance between the pro- and anti-inflammatory roles of the RAS, ultimately causing the proinflammatory role of the RAS to predominate [13], endowing procoagulant properties to endothelial cells [75][245].
