Liver diseases represent a threat to human health and are a significant cause of mortality and morbidity worldwide. Autoimmune hepatitis (AIH) is a progressive and chronic hepatic inflammatory disease, which may lead to severe complications. Concanavalin A (Con A)-induced hepatic injury is regarded as an appropriate experimental model for investigating the pathology and mechanisms involved in liver injury mediated by immune cells as well as T cell-related liver disease. Despite the advances in modern medicine, the only available strategies to treat AIH, include the use of steroids either solely or with immunosuppressant drugs. Unfortunately, this currently available treatment is associated with significant side-effects. Therefore, there is an urgent need for safe and effective drugs to replace and/or supplement those in current use. Natural products have been utilized for treating liver disorders and have become a promising therapy for various liver disorders.
- liver diseases
- autoimmune hepatitis
- inflammation
- concanavalin A
- natural products
- drug discovery
- mechanism of action
1. Introduction
The plant lectin, concanavalin A (Con A) separated from
Canavalia ensiformis
(jack bean) is known as a T-lymphocytes activator
.
T-lymphocytes are effector cells, which play a remarkable role in the immuno-stimulatory process in case of allograft rejection, viral infection, or autoimmune diseases in mammals
[1]
. Con A stimulates T-cell causing a release of several cytokines such as tumor necrosis factor-α (TNF-α), interferon-gamma (IFN-γ)
,
granulocyte macrophage-colony stimulating factor (GM-CSF), and interleukins (ILs), that maintain inflammatory and immuno-stimulatory processes and may arouse acute toxicity
[2]
. Therefore, Con A-activation of T-cell leads to cytokine-induced hepatic injury, which can be assessed by electron microscopy of the liver and by determining the plasma levels of transaminases
[3]
. This injury is characterized by severe liver inflammation and massive hepatocyte apoptosis/necrosis
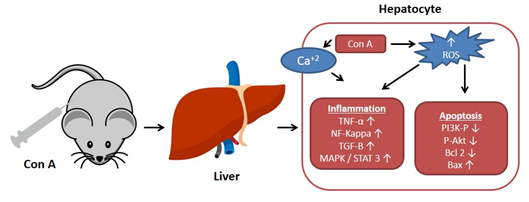
. Con A produces oxidative stress by increasing the ROS levels and decreasing antioxidants levels (e.g., glutathione, SH), which leads to an increase in intracellular Ca
+2
and accelerates lipid peroxidation that damages the cell membrane and other cellular components
. Also, Con A induces inflammation in the hepatic tissue through elevating the levels of TNF-α, adhesion molecules, transforming growth factor-β1 (TGF-β1), mitogen-activated protein kinases (MAPKs), and signal transducer and activator of transcription 3 (STAT3)
. Moreover, apoptosis can be induced by Con A through suppressing Akt (p-Akt), phosphatidylinositol 3 kinase (PI3K), and Bcl2 and upregulating Bax
(Figure 1).
Figure 1.
Effect of concanavalin A (Con A) on the molecular level in Con A-induced hepatotoxicity. NF-kB: Nuclear factor-kappa B; ROS: Reactive oxygen species; TGF-B: Transforming growth factor B; MAPK: Mitogen-activated protein kinase; Stat3: Signal transducer and activator of transcription 3; p-AKT: Phospho-protein kinase B; PI3K: Phosphatidylinositol 3 kinase; TNF-α: Tumor necrosis factor α.
It was reported that Con A-immune responses depend on various cells such as natural killer T (NKT) cells, CD4
+
T cells, neutrophils, and intrahepatic macrophages namely, Kupffer cells (KCs)
[16]
. Con A has been utilized as an insulin receptors agonist and in general T-cell biology
. This is a common model for immune-related hepatitis
[19]
. The pathogenesis in this model is unique and it offers several similarities to acute liver diseases seen in human beings such as: acute liver failure, autoimmune hepatitis (AIH), and acute viral hepatitis in which T-cells involvement and immune activation/infiltration were observed. This model selectively details the T-cell functions in inflammatory liver disease. Therefore, the Con A model is utilized to study the pathogenesis, microscopic morphological changes, and effects of potential treatments for AIH and is recognized as an acceptable and well-characterized model for liver injury mediated by immune responses
.
The liver is a vital organ in nutrients metabolism, immune surveillance, and toxin clearance. However, it can be destroyed by the overactive immune responses that can be triggered by certain medications, metabolic diseases, and toxins, as well as intravascular infections
[22]
. Liver diseases, including viral and autoimmune hepatitis as well as drug-induced liver damage, are a major culprit of mortality and morbidity worldwide and represent a threat to human well-being.
Autoimmune hepatitis (AIH) is a hepatic inflammatory condition, in which the liver parenchyma is destroyed, with lymphocyte infiltration, necrosis, and apoptosis of liver cells, and a rise in the level of transaminase enzymes
. AIH may lead to an array of debilitating complications, including encephalopathy, ascites, cirrhosis, hepatocellular carcinoma, and/or death. The current available management options for AIH include administering steroids (e.g., prednisone, prednisolone) on their own or combining them with immunosuppressive drugs (e.g., azathioprine)
[25]
. However, these medications are non-specific and have significant adverse effects that prompt many patients to discontinue their use
. Thus, there is an urgent need for discovering more safer and specific therapeutic agents.
For hundreds of years, herbal medicines have been utilized for treating liver disorders and have become a promising therapy for various liver disorders
. Many of the therapeutic agents used in liver diseases are either natural products or natural product derivatives because of their capability to act on various biological targets
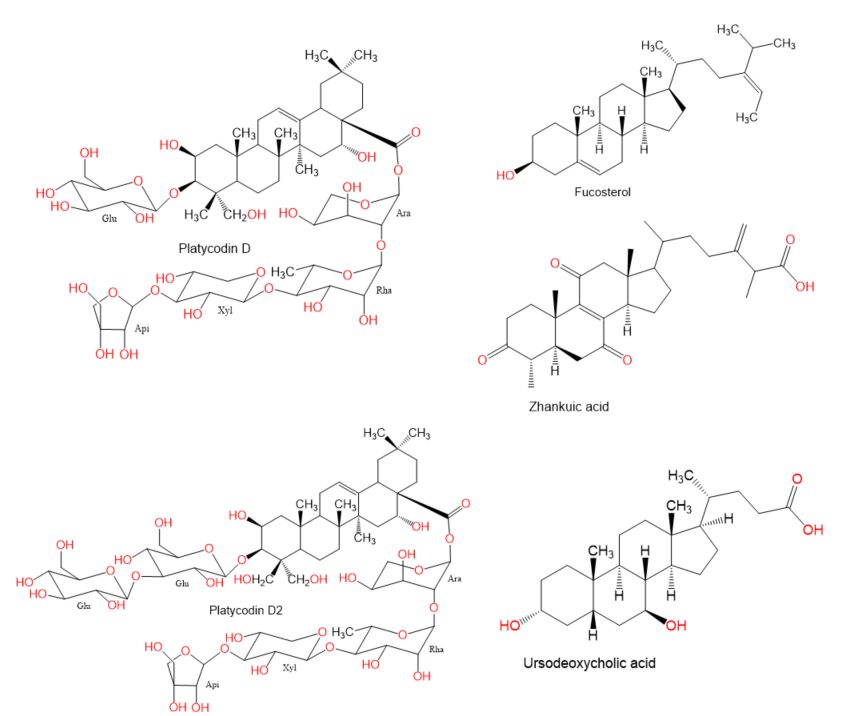
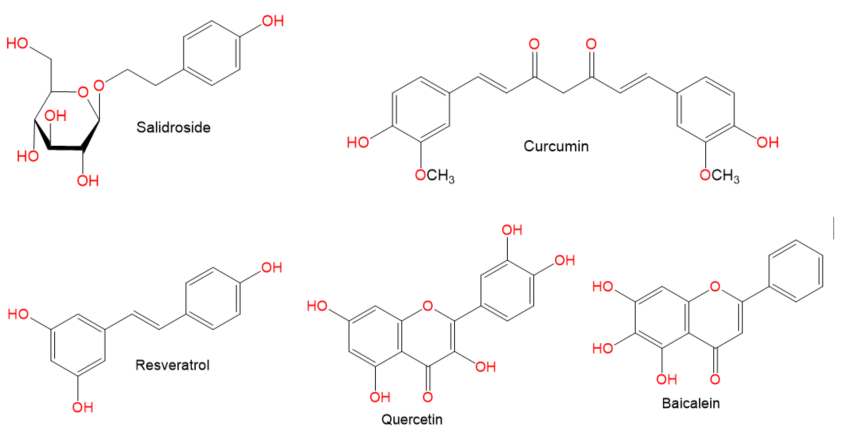
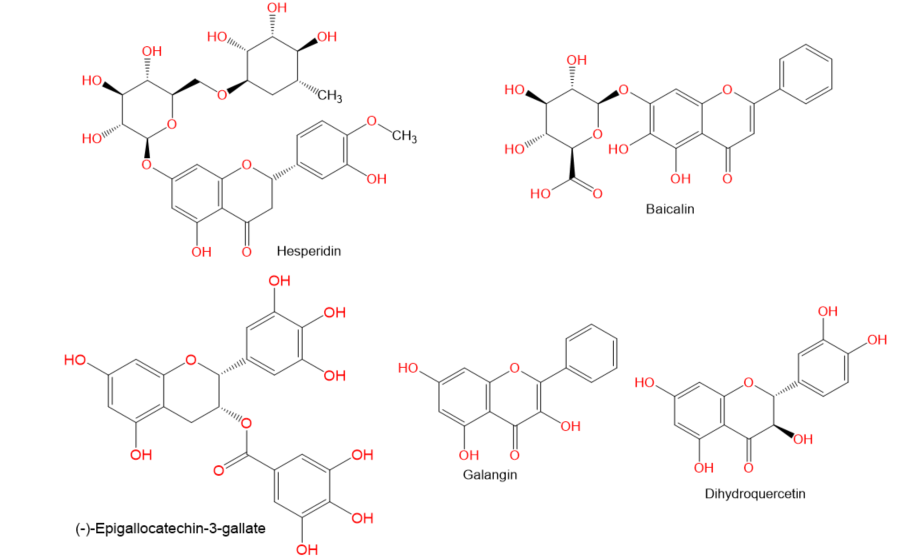
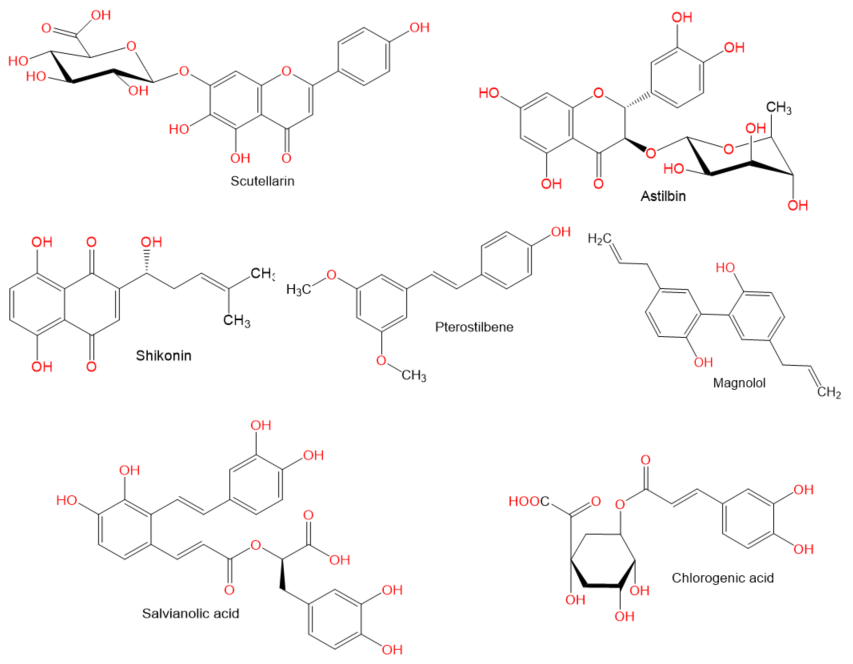
. Moreover, there is a recent expansion of interest in the discovery of natural products from different sources—e.g., terrestrial plants, marine organisms, and microorganisms—as potential leads for treating AIH. In this review, the natural compounds and herbal formulation as well as extracts and/or fractions reported to ameliorate Con A-induced hepatitis and their mechanism of action are reviewed (Figures 2–10; Tables 1–3). This review was performed using the databases; Google Scholar, Science Direct, Springer Link, JACS, Taylor & Francis, Web of Science, Scopus, Bentham Science, or Wiley Online Library. The isolated compounds from different natural sources are classified into different chemical groups. Moreover, their sources, structures, molecular weights, and formulae as well as the effects and possible mechanisms are highlighted (Tables 1 and 2; Figures 2–10). In addition, for plants and herbal formulations, the families, utilized parts, tested fraction, and concentrations were mentioned (Table 3). The current work presents different natural compounds with fascinating skeletons that could be effective for Con A-induced hepatitis. This could attract the interest of pharmacologists and medicinal chemists and offer valuable insights for treating AIH. Therefore, these metabolites could be promising prototype compounds for the discovery of drug candidates that could be used for AIH treatment. Also, this could provide new strategies for immune-mediated liver injury treatment.
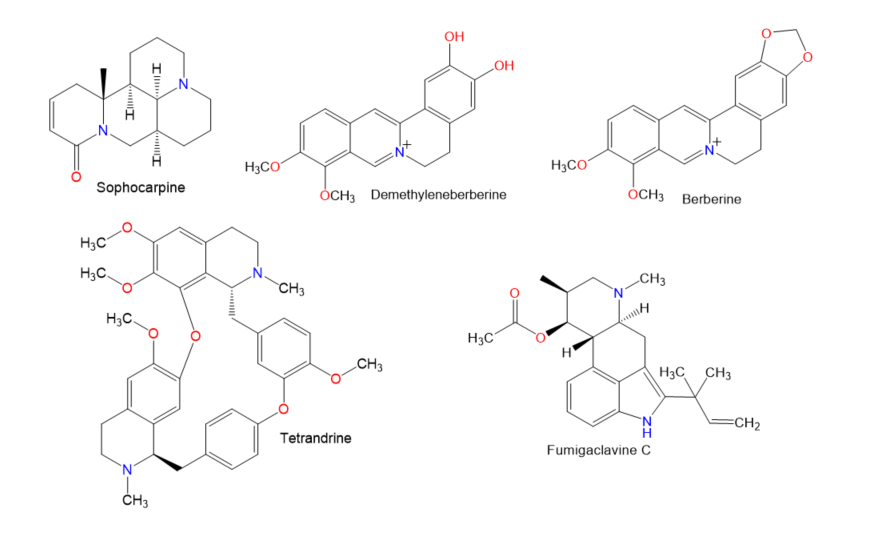
Figure 2.
Chemical structures of alkaloids.
Figure 3.
Chemical structures of alkaloids.
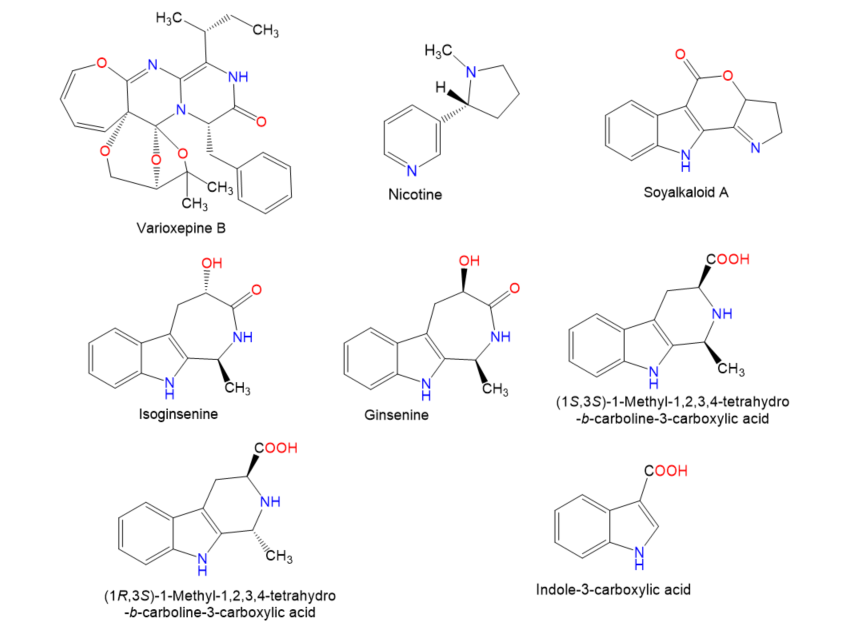
Figure 4.
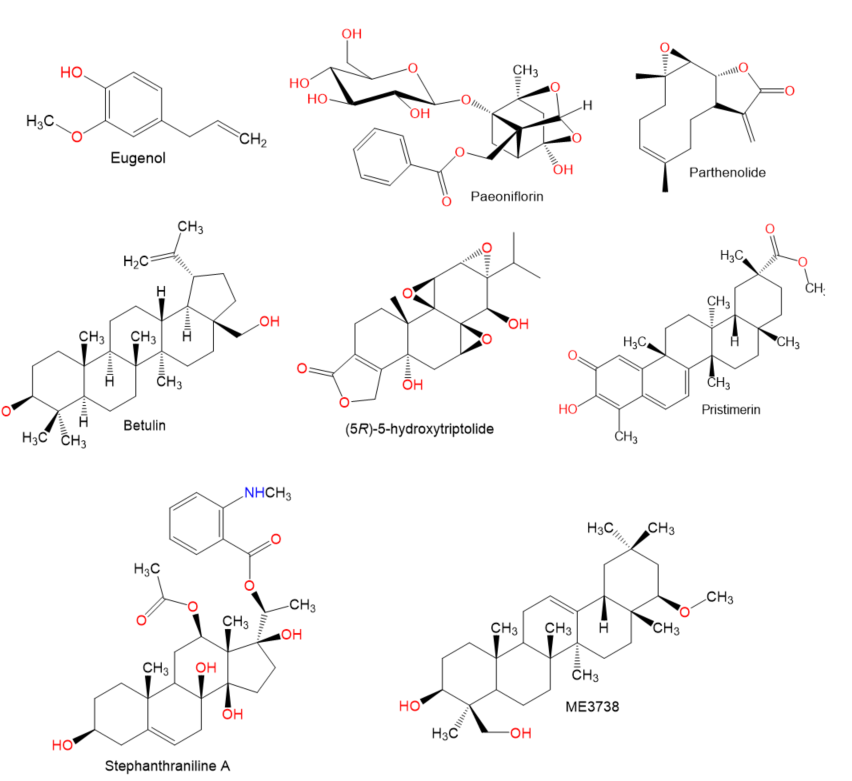
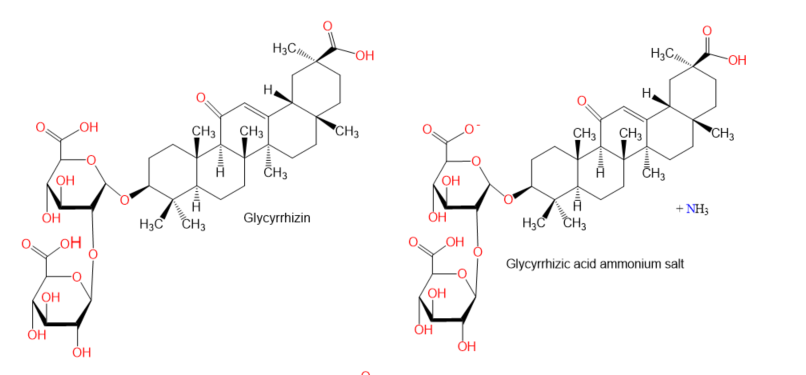
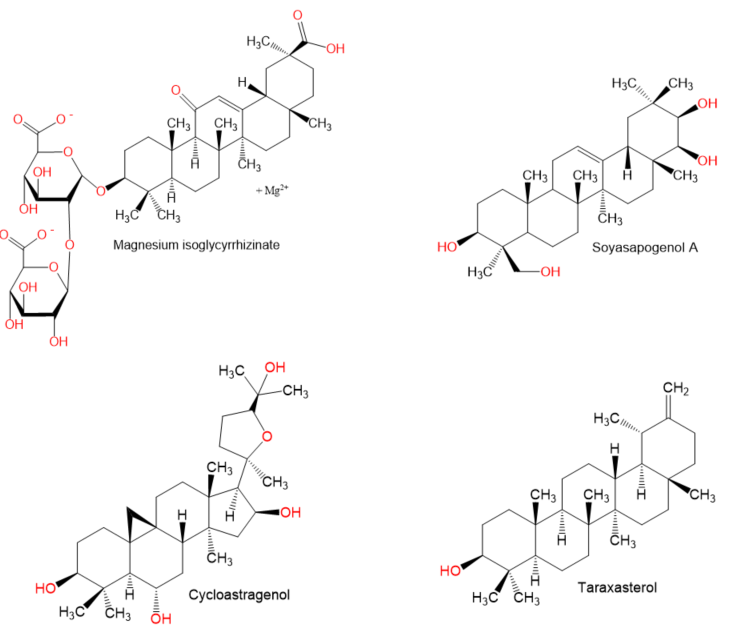
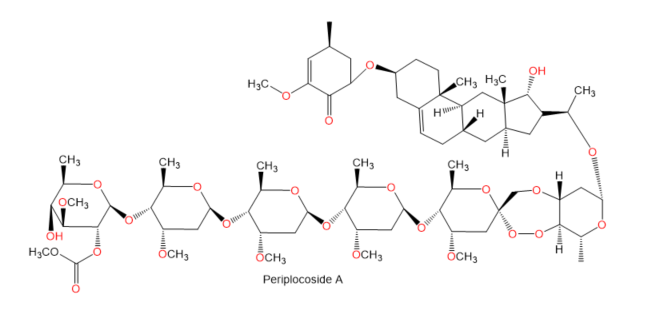
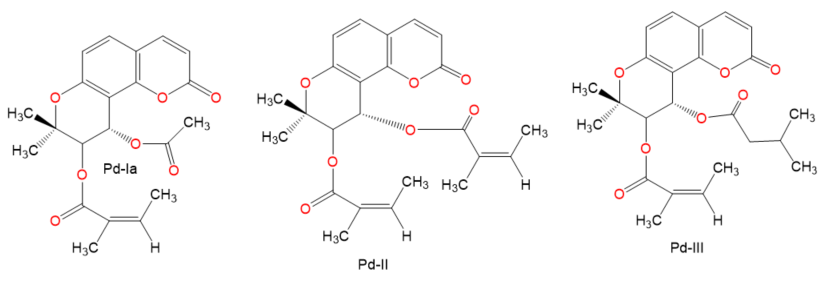
Chemical structures of terpenes.
Figure 5.
Chemical structures of terpenes.
Figure 6.
Chemical structures of terpenes and sterols.
Figure 7.
Chemical structures of phenolics.
Figure 8.
Chemical structures of phenolics.
Figure 9.
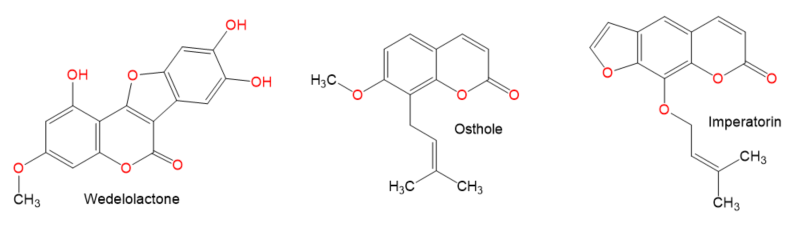
Chemical structures of coumarin and coumarin derivatives.
Figure 10.
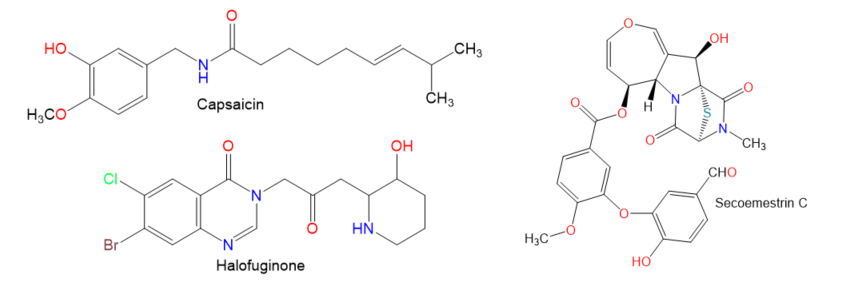
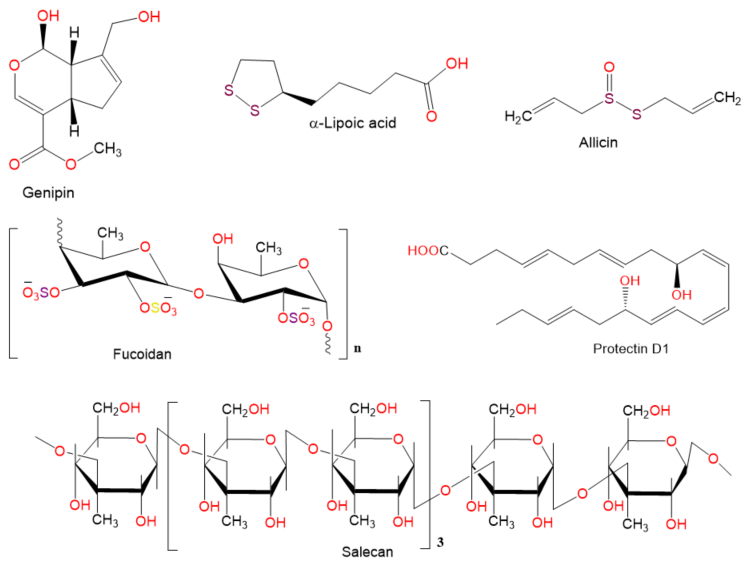
Chemical structures of other compounds.
References
- Gantner, F.; Leist, M.; Lohse, A.W.; Germann, P.G.; Tiegs, G. Concanavalin A-induced T-cell-mediated hepatic injury in mice: The role of tumor necrosis factor. Hepatology 1995, 21, 190–198.
- Miethke, T.; Wahl, C.; Heeg, K.; Echtenacher, B.; Krammer, P.H.; Wagner, H. T cell-mediated lethal shock triggered in mice by the superantigen Staphylococcal enterotoxin B: Critical role of tumor necrosis factor. J. Exp. Med. 1992, 175, 91–98.
- Tiegs, G.; Hentschel, J.; Wendel, A. A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J. Clin. Investig. 1992, 90, 196–203.
- Zhang, H.; Bai, Y.; Gao, M.; Zhang, J.; Dong, G.; Yan, F.; Ma, Q.; Fu, X.; Zhang, Q.; Li, C.; et al. Hepatoprotective effect of capsaicin against concanavalin A-induced hepatic injury via inhibiting oxidative stress and inflammation. Am. J. Transl. Res. 2019, 11, 3029–3038.
- Zhang, Y.; Li, L.; Qi, C.; Hua, S.; Fei, X.; Gong, F.; Fang, M. Glycyrrhizin alleviates Con A-induced hepatitis by differentially regulating the production of IL-17 and IL-25. Biomed. Pharmacother. 2019, 110, 692–699.
- Ballegeer, M.; Libert, C. Different cell types involved in mediating concanavalin A induced liver injury: A comprehensive overview. J. Gastroenterol. Hepatol. Res. 2016, 1, doi:10.24966/GHR-2566/100001.
- Mikkelsen, R.B.; Schmidt-Ullrich, R. Concanavalin A induces the release of intracellular Ca2+ in intact rabbit thymocytes. J. Biol. Chem. 1980, 255, 5177–5183.
- Liu, J.; Mao, Y. Eugenol attenuates concanavalin A-induced hepatitis through modulation of cytokine levels and inhibition of mitochondrial oxidative stress. Arch. Biol. Sci. 2019, 71, 339–346.
- Zhang, M.; Li, Q.; Zhou, C.; Zhao, Y.; Li, R.; Zhang, Y. Demethyleneberberine attenuates concanavalin A-induced autoimmune hepatitis in mice through inhibition of NF-κB and MAPK signaling. Int. Immunopharmacol. 2020, 80, 106137.
- Sang, X.X.; Wang, R.L.; Zhang, C.E.; Liu, S.J.; Shen, H.H.; Guo, Y.M.; Zhang, Y.M.; Niu, M.; Wang, J.B.; Bai, Z.F.; et al. Sophocarpine protects mice from con A-induced hepatitis via inhibition of the IFN-gamma/STAT1 pathway. Front. Pharmacol. 2017, 8, 00140.
- Tu, C.T.; Han, B.; Liu, H.C.; Zhang, S.C. Curcumin protects mice against concanavalin A-induced hepatitis by inhibiting intrahepatic intercellular adhesion molecule-1 (ICAM-1) and CXCL10 expression. Mol. Cell Biochem. 2011, 358, 53–60.
- El-Agamy, D.S.; Shaaban, A.A.; Almaramhy, H.H.; Elkablawy, S.; Elkablawy, M.A. Pristimerin as a novel hepatoprotective agent against experimental autoimmune hepatitis. Front. Pharmacol. 2018, 9, 292.
- Mo, W.; Wang, C.; Li, J.; Chen, K.; Xia, Y.; Li, S.; Xu, L.; Lu, X.; Wang, W.; Guo, C. Fucosterol protects against concanavalin A-induced acute liver injury: Focus on P38 MAPK/NF-κB pathway activity. Gastroenterol Res. Pract. 2018, 2018, 2824139.
- Mao, Y.; Wang, J.; Yu, F.; Cheng, J.; Li, H.; Guo, C.; Fan, X. Ghrelin reduces liver impairment in a model of concanavalin A-induced acute hepatitis in mice. Drug Des. Devel. Ther. 2015, 9, 5385–5396.
- Roy, B.; Pattanaik, A.K.; Das, J.; Bhutia, S.K.; Behera, B.; Singh, P.; Maiti, T.K. Role of PI3K/Akt/mTOR and MEK/ERK pathway in Concanavalin A induced autophagy in HeLa cells. Chem. Biol. Interact. 2014, 210, 96–102.
- Meng, Z.; Wang, J.; Yuan, Y.; Cao, G.; Fan, S.; Gao, C.; Wang, L.; Li, Z.; Wu, X.; Wu, Z.; et al. γδ T cells are indispensable for interleukin-23-mediated protection against Concanavalin A-induced hepatitis in hepatitis B virus transgenic mice. Immunology 2017, 151, 43–55.
- Roth, R.A.; Cassell, D.J.; Maddux, B.A.; Goldfine, I.D. Regulation of insulin receptor kinase activity by insulin mimickers and an insulin antagonist. Biochem. Biophys. Res. Commun. 1983, 115, 245–252.
- Asherson, G.L.; Ferluga, J.; Janossy, G. Non-specific cytotoxicity by T cells activated with plant mitogens in vitro and the requirement for plant agents during the killing reaction. Clin. Exp. Immunol. 1973, 15, 573–589.
- Heymann, F.; Hamesch, K.; Weiskirchen, R.; Tacke, F. The concanavalin A model of acute hepatitis in mice. Lab. Anim. 2015, 49, 12–20.
- Qi, C.; Tan, X.; Shi, Z.; Feng, H.; Sun, L.; Hu, Z.; Chen, G.; Zhang, Y. Discovery of an oxepine-containing diketopiperazine derivative active against concanavalin A-induced hepatitis. J. Nat. Prod. 2020, 83, 2672–2678.
- Wang, Y.; Zhou, L.; Li, Y.; Guo, L.; Zhou, Z.; Xie, H.; Hou, Y.; Wang, B. The effects of berberine on concanavalin A-induced autoimmune hepatitis (AIH) in mice and the adenosine 5’-monophosphate (AMP)-activated protein kinase (AMPK) pathway. Med. Sci. Monit. 2017, 23, 6150–6161.
- Wan, J.; Zhu, Y.N.; Feng, J.Q.; Chen, H.J.; Zhang, R.J.; Ni, J.; Chen, Z.H.; Hou, L.F.; Liu, Q.F.; Zhang, J.; et al. Periplocoside A, a pregnane glycoside from Periploca sepium Bge, prevents concanavalin A-induced mice hepatitis through inhibiting NKT-derived inflammatory cytokine productions. Int. Immunopharmacol. 2008, 8, 1248–1256.
- Yang, Q.; Wang, J.; Liu, R.; Wang, Z.; Li, Y.; Zhang, Y.; Hao, X.; Huang, Y.; Xie, W.; Wei, H. Amelioration of concanavalin A-induced autoimmune hepatitis by magnesium isoglycyrrhizinate through inhibition of CD4(+)CD25(-)CD69(+) subset proliferation. Drug Des. Devel. Ther. 2016, 10, 443–453.
- Manns, M.P.; Lohse, A.W.; Vergani, D. Autoimmune hepatitis—Update 2015. J. Hepatol. 2015, 62, S100–S111.
- Christen, U.; Hintermann, E. Immunopathogenic mechanisms of autoimmune hepatitis: How much do we know from animal models?. Int. J. Mol. Sci. 2016, 17, 2007.
- Christen, U. Animal models of autoimmune hepatitis. Biochim. Biophys. Acta. Mol. Basis Dis. 2019, 1865, 970–981.
- AlSaadi, B.H.; AlHarbi, S.H.; Ibrahim, S.R.M.; El-Kholy, A.A.; El-Agamy, D.S.; Mohamed, G.A. Hepatoprotective activity of Costus speciosus against paracetamol-induced liver injury in mice. Afr. J. Tradit. Complement Altern. Med. 2018, 15, 35–41.
- Syed, S.H.; Namdeo, A.G. Current status of natural products for the treatment of liver disease-A review. Int. J. Phytopharm. 2014, 4, 37–43.
- Zhang, A.; Sun, H.; Wang, X. Recent advances in natural products from plants for treatment of liver diseases. Eur. J. Med. Chem. 2013, 63, 570–577.
- Ibrahim, S.R.M.; El-Agamy, D.S.; Abdallah, H.M.; Ahmed, N.; Elkablawy, M.A.; Mohamed, G.A. Protective activity of tovophyllin A, a xanthone isolated from Garcinia mangostana pericarps, against acetaminophen-induced liver damage: Role of Nrf2 activation. Food Funct. 2018, 9, 3291–3300.