Gynecologic cancers cause over 600,000 deaths annually in women worldwide. The development of chemoresistance after initial rounds of chemotherapy contributes to tumor relapse and death due to gynecologic malignancies. In this regard, cancer stem cells (CSCs), a subpopulation of stem cells with the ability to undergo self-renewal and clonal evolution, play a key role in tumor progression and drug resistance. Aldehyde dehydrogenases (ALDH) are a group of enzymes shown to be robust CSC markers in gynecologic and other malignancies. These enzymes also play functional roles in CSCs, including detoxification of aldehydes, scavenging of reactive oxygen species (ROS), and retinoic acid (RA) signaling, making ALDH an attractive therapeutic target in various clinical scenarios. In this review, we discuss the critical roles of the ALDH in driving stemness in different gynecologic malignancies. We review inhibitors of ALDH, both general and isoform-specific, which have been used to target CSCs in gynecologic cancers. Many of these inhibitors have been shown to be effective in preclinical models of gynecologic malignancies, supporting further development in the clinic. Furthermore, ALDH inhibitors, including 673A and CM037, synergize with chemotherapy to reduce tumor growth. Thus, ALDH-targeted therapies hold promise for improving patient outcomes in gynecologic malignancies.
- gynecologic malignancies
- cancer stem cells
- aldehyde dehydrogenases
Note: The following contents are extracted from your paper. The entry will be online only after author check and submit it.
1. Introduction
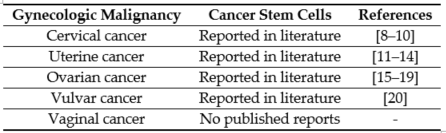
The first line of therapy for most gynecologic cancers includes surgery, followed by chemotherapy and radiation [1]. However, in the majority of cases, these conventional therapies do not completely eliminate the malignant cells. The primary reason for high mortality is recurrence and subsequent metastasis caused by the residual population of cancer cells [2,3][2][3]. The cells that survive after the first line of treatment and contribute to cancer recurrence are known as CSCs [4,5][4][5]. The CSC theory states that the tumor is a heterogeneous mass, and within the tumor exists a hierarchy of cells, with CSCs at the apex [6]. Lapidot et al. first proposed the idea that a set of specialized cells present within the tumor can sustain and repopulate the tumor [7] [7]. CSCs have since been reported in gynecologic malignancies (Table 1).
Table 1. Cancer stem cells reported in gynecologic malignancies.[8][9][10][11][12][13][14][15][16][17][18][19][20]
CSCs are resistant to conventional chemotherapy due to several mechanisms. Chemotherapeutic drugs, primarily platinum-based drugs, form DNA crosslinks, killing cells by causing DNA damage in rapidly-dividing cells [21]. However, CSCs are resistant to DNA damage due to a number of properties, including slow cycling, reduced uptake of drugs and increased drug efflux due to the high expression of a class of non-selective drug transporters called adenosine triphosphate binding cassette (ABC) ATPases [22]. Furthermore, CSCs have enhanced DNA repair due to overexpression of repair pathways such as ataxia-telangiectasia-mutated (ATM), ataxia telangiectasia and rad3-related (ATR), checkpoint kinase 1 (Chk1), poly(ADP-ribose) polymerase 1 (PARP1), and RAD51 [23] that protect CSCs from drugs designed to cause cancer cell death by inducing DNA damage. As a quiescent population [24], CSCs are further protected by platinum-induced DNA damage. Thus, it is necessary to target CSCs specifically to achieve a better prognosis in patients. Of the different CSC markers identified to date in gynecologic malignancies [10–20,22,23], ALDH is widely recognized as a highly robust CSC marker across the vast majority of cancer types, including gynecologic CSCs. Furthermore, ALDH holds the distinction of having potential functional importance in the maintenance of CSCs [25], making it an attractive target for eradicating CSC in the therapeutic maintenance setting for gynecologic malignancies such as ovarian cancer.
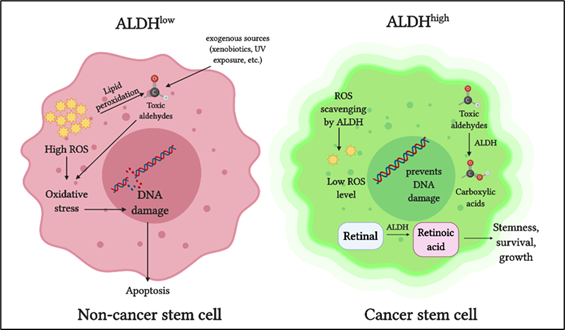
The ALDH superfamily comprises 19 members, all of which are involved in regulating crucial functions in normal as well as cancer stem cells [13–19]. The primary role of ALDH enzymes is to metabolize reactive aldehydes produced by various biological processes [26] (Figure 1).
Figure 1. Role of aldehyde dehydrogenases (ALDH) in cancer stem cells: ALDH detoxifies toxic aldehydes (endogenous and exogenous) into less toxic carboxylic acids. ALDH maintains intracellular reactive oxygen species (ROS) at a low level thus preventing oxidative stress and DNA damage. ALDH oxidizes retinaldehyde into retinoic acid, which promotes stemness, growth, and survival in cancer stem cells.
Detoxification of aldehydes is critical for cellular health, as aldehyde toxicity can lead to DNA damage, impaired cellular homeostasis, and cell death [27]. Another vital role of ALDH is in retinoic acid metabolism, which is crucial for gene expression and morphogenesis during embryonic development growth, cellular differentiation, and homeostasis of vertebrates [28–30][28][29][30]. Cytosolic class I ALDH enzymes catalyze the NAD‐dependent oxidation of both all‐trans‐retinal and 9‐cis‐retinal to all‐trans‐retinoic acid and 9‐cis‐retinoic acid [31,32][31][32]. ALDH also plays a role in reactive oxygen species (ROS) scavenging and thereby reducing oxidative stress in stem cells [33] (Figure 1).
In cancer cells, ALDH contributes to chemoresistance via different mechanisms [34]. ALDH isoforms, ALDH1A1, and ALDH3A1 are both involved in the metabolism of the cancer drug cyclophosphamide, metabolizing the active compound to a less active form and contributing to drug resistance [35]. When combined with cyclophosphamide in ALDH3A1high cell lines, ALDH3A1 inhibitors have been shown to increase sensitivity to the mafosphamide (cyclophosphamide analog) [34]. Another mechanism by which ALDH protects cancer cells is by reducing ROS-mediated oxidative stress [36] (Figure 1). As a consequence of this, ALDHhigh CSCs have a lower baseline ROS level and oxidative damage than the ALDHlow counterparts [33].
Clinically, high ALDH expression is associated with poor outcomes in several gynecologic malignancies, including ovarian cancer (OC) [17[37][38][39][40],37–40], endometrial [41], and cervical cancer (CC) [42[42][43],43], as well as other solid tumors including breast [44–46][44][45][46], lung adenocarcinoma [47], rectal [48], esophageal squamous adenocarcinoma [49], gastric [50], colorectal [51], prostate [52], and neuroblastoma [53]. To our knowledge, there are no published reports correlating ALDH and prognosis in vulvar or vaginal cancer. Of the 19 ALDH isoforms, ALDH1 is the primary isoform implicated in CSCs of solid tumors [54]. The advent of the Aldefluor assay has stimulated research on CSCs expressing high ALDH. Briefly, the Aldefluor assay can be used to detect cells expressing high levels of ALDH. The Aldefluor reagent Bodipy- aminoacetaldehyde (BAAA) is converted into BODIPY-aminoacetate (BAA), in the presence of ALDH, and retained inside the cells. ALDH activity of the cells is directly proportional to the fluorescence intensity [55]. The assay can detect nine ALDH isoforms, with ALDH1 as the predominant isoform contributing to ALDHhigh cells [56]. These provide a strong rationale for targeting ALDH to eliminate CSCs in gynecologic malignancies. A recent review by Dinavahi et al. elegantly highlights the inhibitors developed to target ALDH from a pharmacologic perspective in different cancer types [57].
2. ALDH in Gynecologic Cancers
2.1. ALDH and Cervical Cancer
In tissue specimens from patients with cervical SCC or cervical intraepithelial neoplasia (CIN) II-III, high ALDH expression was observed immunohistochemically [43]. Interestingly, peripheral blood (plasma) samples from the same patients had increased ALDH1A1 expression when compared with samples from healthy patients [43]. In patients with invasive SCC, ALDH1 expression correlated with lymph nodal metastasis and disease recurrence [69][58]. These data indicate that ALDH1 can be used as a reliable biomarker for the identification of cervical CSCs [70][59]. The ALDHhigh cells isolated from these cancers showed high gene and protein expression of stemness transcription factors Nanog, sex-determining region Y-box2 (Sox2), octamer-binding transcription factor (Oct4), and twist-related protein 1 (Twist1) [43]. However, the exact mechanism by which ALDH regulates stemness in cervical cancer remains incompletely understood.
2.2. ALDH and Uterine Cancer
In EC, the most prevalent type of uterine cancer, altered stemness-related pathways, including Wnt and β-catenin, support a role for CSCs [71][60]. Furthermore, ALDHhigh subpopulation of cells has been demonstrated to be CSCs in EC [71][60]. The ALDHhigh cells isolated from primary endometrial tumors were highly tumorigenic and resistant to chemotherapeutic drugs and showed increased invasive ability compared to the ALDHlow cells [72][61].
In patients with uterine endometrioid carcinosarcoma, high ALDH1 expression predicted poor prognosis, lymphatic invasion, recurrence, and low overall survival [73][62]. ALDHhigh CSCs cells have distinct stem-like properties, such as high expression of stem-cell markers BMI1, HEY1, HES1, and adhesive molecule CD44 [41,74,75][63][64]; in addition, reduced expression of differentiation markers, enhanced migration, high tumorigenicity, and self-renewal ability was reported [76][65]. When EC cells were sorted using flow cytometry and cultured in vitro, ALDHhigh cells yielded both ALDHhigh and ALDHlow cells, whereas ALDHlow cells only yielded ALDHlow cells [72]. These results demonstrated that endometrial CSCs divide asymmetrically, in agreement with the CSC hypothesis. Furthermore, when injected into mice subcutaneously, ALDHhigh endometrial cells formed larger tumors more rapidly than the ALDHlow cells [72], demonstrating that CSCs were able to repopulate the entire endometrial tumor mass. Based on these studies ALDH1 serves as both a marker for identifying endometrial CSCs and a therapeutic target, based in its functional importance in the disease.
2.3. ALDH and Ovarian Cancer
Cancer relapse after surgery and chemotherapy is common in OC, and CSCs are strongly associated with OC relapse [77] [66]. ALDHhigh cells are widely accepted as CSCs in OC, as demonstrated by us and others [17,37–39,78–83][67][68][69][70][71][72]. ALDHhigh ovarian cancer stem cells (OCSCs) exhibit classic stem cell characteristics, such as being highly chemoresistant and enriched in residual xenografts after platinum therapy [78,79,83][72]. Upregulation of stemness genes such as Sox2, Kruppel like factor 4 (Klf4), Nanog, and downregulation of differentiation genes such as homeobox A10 (HOXA10) and homeobox A11 (HOXA11), were reported in ALDHhigh cells [83][72]. ALDHhigh cells demonstrated enhanced ability to form spheroids in low attachment conditions in vitro [75,80].
In OCSCs, of the 19 ALDH isoforms, ALDH1A1 was highly correlated with chemotherapy resistance [78,79,81,82,84,85][73][74]. Expression of ALDH1A1 was 100-fold higher in OC cells selected for taxane-resistance in vitro, and ALDH1A1 knockdown sensitized the resistant cells to chemotherapy [82]. ALDH1A1 expression was higher in residual tumors after the first round of chemotherapy compared to tumors from untreated patients [86][75], demonstrating enrichment of OCSC post-treatment. In addition to ALDH1A1, CD133 serves as a robust marker for OCSC when used in combination with ALDH [17]. In this regard, Silva et al. observed that as low as 11 ALDH and CD133 double-positive cells resulted in tumor induction in mice [17]. Moreover, in tumors harvested during debulking surgeries, ALDHhighCD133+ cells correlated with reduced disease-free and overall survival in OC patients [17]. ALDHhigh cells were highly metastatic with the enhanced invasive ability and were resistant to apoptosis [87] [76]. These data provide a strong rationale for targeting ALDHhigh cells in OC [37].
2.4. ALDH and Vulvar Cancer
Vulvar cancer is an uncommon type of tumor in women. To date, one study on ALDH in this gynecologic cancer has been reported. ALDH1 expression in vulvar squamous cell carcinoma, normal vulvar epithelium, and stromal tissues in a cohort of 154 patients was studied [88][77]. Based on clinicopathological studies, high ALDH1 expression correlated with a favorable prognosis and can be considered a potential marker for differentiated vulvar cells [88][77]. However, this report is contradictory to the correlation of ALDH with poor prognosis in other gynecologic cancers, suggesting that ALDH expression could be a tissue-specific marker for CSCs.
References
- Arruebo, M.; Vilaboa, N.; Saez-Gutierrez, B.; Lambea, J.; Tres, A.; Valladares, M.; Gonzalez-Fernandez, A. Assessment of the evolution of cancer treatment therapies. Cancers 2011, 3, 3279–3330, doi:10.3390/cancers3033279.
- Vaz-Luis, I.; Lin, N.U.; Keating, N.L.; Barry, W.T.; Winer, E.P.; Freedman, R.A. Factors Associated with Early Mortality Among Patients with De Novo Metastatic Breast Cancer: A Population-Based Study. Oncologist 2017, 22, 386–393, doi:10.1634/theoncologist.2016-0369.
- Grasic-Kuhar, C.; Bracko, M.; Zakotnik, B. Risk factors for late relapse and death in patients with early breast cancer. Neoplasma 2008, 55, 416–420.
- Galluzzi, L.; Senovilla, L.; Vitale, I.; Michels, J.; Martins, I.; Kepp, O.; Castedo, M.; Kroemer, G. Molecular mechanisms of cisplatin resistance. Oncogene 2012, 31, 1869–1883, doi:10.1038/onc.2011.384.
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111, doi:10.1038/35102167.
- Visvader, J.E.; Lindeman, G.J. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nat. Rev. Cancer 2008, 8, 755–768, doi:10.1038/nrc2499.
- Lapidot, T.; Sirard, C.; Vormoor, J.; Murdoch, B.; Hoang, T.; Caceres-Cortes, J.; Minden, M.; Paterson, B.; Caligiuri, M.A.; Dick, J.E. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994, 367, 645–648, doi:10.1038/367645a0.
- Villanueva-Toledo, J.; Ponciano-Gomez, A.; Ortiz-Sanchez, E.; Garrido, E. Side populations from cervical-cancer-derived cell lines have stem-cell-like properties. Mol. Biol. Rep. 2014, 41, 1993–2004, doi:10.1007/s11033-014-3047-3.
- Liu, S.Y.; Zheng, P.S. High aldehyde dehydrogenase activity identifies cancer stem cells in human cervical cancer. Oncotarget 2013, 4, 2462–2475, doi:10.18632/oncotarget.1578.
- Kumazawa, S.; Kajiyama, H.; Umezu, T.; Mizuno, M.; Suzuki, S.; Yamamoto, E.; Mitsui, H.; Sekiya, R.; Shibata, K.; Kikkawa, F. Possible association between stem-like hallmark and radioresistance in human cervical carcinoma cells. J. Obstet. Gynaecol. Res. 2014, 40, 1389–1398, doi:10.1111/jog.12357.
- Gorai, I.; Yanagibashi, T.; Taki, A.; Udagawa, K.; Miyagi, E.; Nakazawa, T.; Hirahara, F.; Nagashima, Y.; Minaguchi, H. Uterine carcinosarcoma is derived from a single stem cell: An in vitro study. Int. J. Cancer 1997, 72, 821–827, doi:10.1002/(sici)1097-0215(19970904)72:5<821::aid-ijc19>3.0.co;2-b.
- Gotte, M.; Greve, B.; Kelsch, R.; Muller-Uthoff, H.; Weiss, K.; Kharabi Masouleh, B.; Sibrowski, W.; Kiesel, L.; Buchweitz, O. The adult stem cell marker Musashi-1 modulates endometrial carcinoma cell cycle progression and apoptosis via Notch-1 and p21WAF1/CIP1. Int. J. Cancer 2011, 129, 2042–2049, doi:10.1002/ijc.25856.
- Rutella, S.; Bonanno, G.; Procoli, A.; Mariotti, A.; Corallo, M.; Prisco, M.G.; Eramo, A.; Napoletano, C.; Gallo, D.; Perillo, A.; et al. Cells with characteristics of cancer stem/progenitor cells express the CD133 antigen in human endometrial tumors. Clin. Cancer Res. 2009, 15, 4299–4311, doi:10.1158/1078-0432.CCR-08-1883.
- Kato, K.; Takao, T.; Kuboyama, A.; Tanaka, Y.; Ohgami, T.; Yamaguchi, S.; Adachi, S.; Yoneda, T.; Ueoka, Y.; Kato, K.; et al. Endometrial cancer side-population cells show prominent migration and have a potential to differentiate into the mesenchymal cell lineage. Am. J. Pathol. 2010, 176, 381–392, doi:10.2353/ajpath.2010.090056.
- Zhang, S.; Balch, C.; Chan, M.W.; Lai, H.C.; Matei, D.; Schilder, J.M.; Yan, P.S.; Huang, T.H.; Nephew, K.P. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008, 68, 4311–4320, doi:10.1158/0008-5472.CAN-08-0364.
- Shi, M.F.; Jiao, J.; Lu, W.G.; Ye, F.; Ma, D.; Dong, Q.G.; Xie, X. Identification of cancer stem cell-like cells from human epithelial ovarian carcinoma cell line. Cell Mol. Life Sci. 2010, 67, 3915–3925, doi:10.1007/s00018-010-0420-9.
- Silva, I.A.; Bai, S.; McLean, K.; Yang, K.; Griffith, K.; Thomas, D.; Ginestier, C.; Johnston, C.; Kueck, A.; Reynolds, R.K.; et al. Aldehyde dehydrogenase in combination with CD133 defines angiogenic ovarian cancer stem cells that portend poor patient survival. Cancer Res. 2011, 71, 3991–4001, doi:10.1158/0008-5472.CAN-10-3175.
- Bapat, S.A.; Mali, A.M.; Koppikar, C.B.; Kurrey, N.K. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res. 2005, 65, 3025–3029, doi:10.1158/0008-5472.CAN-04-3931.
- Gao, M.Q.; Choi, Y.P.; Kang, S.; Youn, J.H.; Cho, N.H. CD24+ cells from hierarchically organized ovarian cancer are enriched in cancer stem cells. Oncogene 2010, 29, 2672–2680, doi:10.1038/onc.2010.35.
- Napoletano, C.; Bellati, F.; Ruscito, I.; Pernice, M.; Zizzari, I.G.; Caponnetto, S.; Tomao, F.; Frigerio, L.; Liberati, M.; Rughetti, A.; et al. Immunological and Clinical Impact of Cancer Stem Cells in Vulvar Cancer: Role of CD133/CD24/ABCG2-Expressing Cells. Anticancer Res. 2016, 36, 5109–5116, doi:10.21873/anticanres.11080.
- Dubos, R.J. The Mode of Action of Chemotherapeutic Agents. Bull. N.Y. Acad. Med. 1945, 21, 27–36.
- Vermeulen, L.; Sprick, M.R.; Kemper, K.; Stassi, G.; Medema, J.P. Cancer stem cells—Old concepts, new insights. Cell Death Differ. 2008, 15, 947–958, doi:10.1038/cdd.2008.20.
- Yu, W.K.; Wang, Z.; Fong, C.C.; Liu, D.; Yip, T.C.; Au, S.K.; Zhu, G.; Yang, M. Chemoresistant lung cancer stem cells display high DNA repair capability to remove cisplatin-induced DNA damage. Br. J. Pharmacol. 2017, 174, 302–313, doi:10.1111/bph.13690.
- Zhou, J.; Zhang, Y. Cancer stem cells: Models, mechanisms and implications for improved treatment. Cell Cycle 2008, 7, 1360–1370, doi:10.4161/cc.7.10.5953.
- Vassalli, G. Aldehyde Dehydrogenases: Not Just Markers, but Functional Regulators of Stem Cells. Stem Cells Int 2019, 2019, 3904645, doi:10.1155/2019/3904645.
- Pors, K.; Moreb, J.S. Aldehyde dehydrogenases in cancer: An opportunity for biomarker and drug development? Drug Discov. Today 2014, 19, 1953–1963, doi:10.1016/j.drudis.2014.09.009.
- Mizumoto, A.; Ohashi, S.; Hirohashi, K.; Amanuma, Y.; Matsuda, T.; Muto, M. Molecular Mechanisms of Acetaldehyde-Mediated Carcinogenesis in Squamous Epithelium. Int. J. Mol. Sci. 2017, 18, doi:10.3390/ijms18091943.
- Blomhoff, R. Transport and metabolism of vitamin A. Nutr. Rev. 1994, 52, S13–23, doi:10.1111/j.1753-4887.1994.tb01382.x.
- Chanda, B.; Ditadi, A.; Iscove, N.N.; Keller, G. Retinoic acid signaling is essential for embryonic hematopoietic stem cell development. Cell 2013, 155, 215–227, doi:10.1016/j.cell.2013.08.055.
- Appel, B.; Eisen, J.S. Retinoids run rampant: Multiple roles during spinal cord and motor neuron development. Neuron 2003, 40, 461–464.
- Labrecque, J.; Dumas, F.; Lacroix, A.; Bhat, P.V. A novel isoenzyme of aldehyde dehydrogenase specifically involved in the biosynthesis of 9-cis and all-trans retinoic acid. Biochem. J. 1995, 305, 681-684, doi:10.1042/bj3050681.
- Wald, G. The chemistry of rod vision. Science 1951, 113, 287–291, doi:10.1126/science.113.2933.287.
- Singh, S.; Brocker, C.; Koppaka, V.; Chen, Y.; Jackson, B.C.; Matsumoto, A.; Thompson, D.C.; Vasiliou, V. Aldehyde dehydrogenases in cellular responses to oxidative/electrophilic stress. Free Radic. Biol. Med. 2013, 56, 89–101, doi:10.1016/j.freeradbiomed.2012.11.010.
- Parajuli, B.; Fishel, M.L.; Hurley, T.D. Selective ALDH3A1 inhibition by benzimidazole analogues increase mafosfamide sensitivity in cancer cells. J. Med. Chem. 2014, 57, 449–461, doi:10.1021/jm401508p.
- Hilton, J. Role of aldehyde dehydrogenase in cyclophosphamide-resistant L1210 leukemia. Cancer Res. 1984, 44, 5156–5160.
- Ikeda, J.; Mamat, S.; Tian, T.; Wang, Y.; Luo, W.; Rahadiani, N.; Aozasa, K.; Morii, E. Reactive oxygen species and aldehyde dehydrogenase activity in Hodgkin lymphoma cells. Lab. Invest. 2012, 92, 606–614, doi:10.1038/labinvest.2012.4.
- Xia, Y.; Wei, X.; Gong, H.; Ni, Y. Aldehyde dehydrogenase serves as a biomarker for worse survival profiles in ovarian cancer patients: An updated meta-analysis. BMC Womens Health 2018, 18, 199, doi:10.1186/s12905-018-0686-x.
- Kuroda, T.; Hirohashi, Y.; Torigoe, T.; Yasuda, K.; Takahashi, A.; Asanuma, H.; Morita, R.; Mariya, T.; Asano, T.; Mizuuchi, M.; et al. ALDH1-high ovarian cancer stem-like cells can be isolated from serous and clear cell adenocarcinoma cells, and ALDH1 high expression is associated with poor prognosis. PLoS ONE 2013, 8, e65158, doi:10.1371/journal.pone.0065158.
- Ayub, T.H.; Keyver-Paik, M.D.; Debald, M.; Rostamzadeh, B.; Thiesler, T.; Schroder, L.; Barchet, W.; Abramian, A.; Kaiser, C.; Kristiansen, G.; et al. Accumulation of ALDH1-positive cells after neoadjuvant chemotherapy predicts treatment resistance and prognosticates poor outcome in ovarian cancer. Oncotarget 2015, 6, 16437–16448, doi:10.18632/oncotarget.4103.
- Sun, Y.; Jia, X.; Wu, X. High Expressions of Lgr5 and ALDH1 in Primary Epithelial Ovarian Cancer Correlate with Advanced Tumor Stage and Grade as well as Poor Prognosis of the Patients. Gynecol. Obstet. Invest. 2015, doi:10.1159/000431222.
- Huang, H.H.; Wang, Y.C.; Chou, Y.C.; Yu, M.H.; Chao, T.K. The combination of aldehyde dehydrogenase 1 (ALDH1) and CD44 is associated with poor outcomes in endometrial cancer. PLoS ONE 2018, 13, e0206685, doi:10.1371/journal.pone.0206685.
- Yao, T.; Wu, Z.; Liu, Y.; Rao, Q.; Lin, Z. Aldehyde dehydrogenase 1 (ALDH1) positivity correlates with poor prognosis in cervical cancer. J. Int. Med. Res. 2014, 42, 1038–1042, doi:10.1177/0300060514527060.
- Tulake, W.; Yuemaier, R.; Sheng, L.; Ru, M.; Lidifu, D.; Abudula, A. Upregulation of stem cell markers ALDH1A1 and OCT4 as potential biomarkers for the early detection of cervical carcinoma. Oncol. Lett. 2018, 16, 5525–5534, doi:10.3892/ol.2018.9381.
- Ginestier, C.; Hur, M.H.; Charafe-Jauffret, E.; Monville, F.; Dutcher, J.; Brown, M.; Jacquemier, J.; Viens, P.; Kleer, C.G.; Liu, S.; et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007, 1, 555–567, doi:10.1016/j.stem.2007.08.014.
- Balicki, D. Moving forward in human mammary stem cell biology and breast cancer prognostication using ALDH1. Cell Stem Cell 2007, 1, 485–487, doi:10.1016/j.stem.2007.10.015.
- Neumeister, V.; Agarwal, S.; Bordeaux, J.; Camp, R.L.; Rimm, D.L. In situ identification of putative cancer stem cells by multiplexing ALDH1, CD44, and cytokeratin identifies breast cancer patients with poor prognosis. Am. J. Pathol. 2010, 176, 2131–2138, doi:10.2353/ajpath.2010.090712.
- Sullivan, J.P.; Spinola, M.; Dodge, M.; Raso, M.G.; Behrens, C.; Gao, B.; Schuster, K.; Shao, C.; Larsen, J.E.; Sullivan, L.A.; et al. Aldehyde dehydrogenase activity selects for lung adenocarcinoma stem cells dependent on notch signaling. Cancer Res. 2010, 70, 9937–9948, doi:10.1158/0008-5472.CAN-10-0881.
- Deng, Y.; Zhou, J.; Fang, L.; Cai, Y.; Ke, J.; Xie, X.; Huang, Y.; Huang, M.; Wang, J. ALDH1 is an independent prognostic factor for patients with stages II-III rectal cancer after receiving radiochemotherapy. Br. J. Cancer 2014, 110, 430–434, doi:10.1038/bjc.2013.767.
- Wang, Y.; Zhe, H.; Gao, P.; Zhang, N.; Li, G.; Qin, J. Cancer stem cell marker ALDH1 expression is associated with lymph node metastasis and poor survival in esophageal squamous cell carcinoma: A study from high incidence area of northern China. Dis. Esophagus 2012, 25, 560–565, doi:10.1111/j.1442-2050.2011.01279.x.
- Li, X.S.; Xu, Q.; Fu, X.Y.; Luo, W.S. ALDH1A1 overexpression is associated with the progression and prognosis in gastric cancer. BMC Cancer 2014, 14, 705, doi:10.1186/1471-2407-14-705.
- Van der Waals, L.M.; Borel Rinkes, I.H.M.; Kranenburg, O. ALDH1A1 expression is associated with poor differentiation, 'right-sidedness' and poor survival in human colorectal cancer. PLoS ONE 2018, 13, e0205536, doi:10.1371/journal.pone.0205536.
- Van den Hoogen, C.; van der Horst, G.; Cheung, H.; Buijs, J.T.; Lippitt, J.M.; Guzman-Ramirez, N.; Hamdy, F.C.; Eaton, C.L.; Thalmann, G.N.; Cecchini, M.G.; et al. High aldehyde dehydrogenase activity identifies tumor-initiating and metastasis-initiating cells in human prostate cancer. Cancer Res. 2010, 70, 5163–5173, doi:10.1158/0008-5472.CAN-09-3806.
- Flahaut, M.; Jauquier, N.; Chevalier, N.; Nardou, K.; Balmas Bourloud, K.; Joseph, J.M.; Barras, D.; Widmann, C.; Gross, N.; Renella, R.; et al. Aldehyde dehydrogenase activity plays a Key role in the aggressive phenotype of neuroblastoma. BMC Cancer 2016, 16, 781, doi:10.1186/s12885-016-2820-1.
- Ruscito, I.; Darb-Esfahani, S.; Kulbe, H.; Bellati, F.; Zizzari, I.G.; Rahimi Koshkaki, H.; Napoletano, C.; Caserta, D.; Rughetti, A.; Kessler, M.; et al. The prognostic impact of cancer stem-like cell biomarker aldehyde dehydrogenase-1 (ALDH1) in ovarian cancer: A meta-analysis. Gynecol. Oncol. 2018, 150, 151–157, doi:10.1016/j.ygyno.2018.05.006.
- Storms, R.W.; Trujillo, A.P.; Springer, J.B.; Shah, L.; Colvin, O.M.; Ludeman, S.M.; Smith, C. Isolation of primitive human hematopoietic progenitors on the basis of aldehyde dehydrogenase activity. Proc. Natl. Acad. Sci. USA 1999, 96, 9118–9123, doi:10.1073/pnas.96.16.9118.
- Zhou, L.; Sheng, D.; Wang, D.; Ma, W.; Deng, Q.; Deng, L.; Liu, S. Identification of cancer-type specific expression patterns for active aldehyde dehydrogenase (ALDH) isoforms in ALDEFLUOR assay. Cell Biol. Toxicol. 2019, 35, 161–177, doi:10.1007/s10565-018-9444-y.
- Dinavahi, S.S.; Bazewicz, C.G.; Gowda, R.; Robertson, G.P. Aldehyde Dehydrogenase Inhibitors for Cancer Therapeutics. Trends Pharmacol. Sci. 2019, 40, 774–789, doi:10.1016/j.tips.2019.08.002.
- Yao, T.; Chen, Q.; Zhang, B.; Zhou, H.; Lin, Z. The expression of ALDH1 in cervical carcinoma. Med. Sci. Monit. 2011, 17, HY21–HY26, doi:10.12659/msm.881886.
- Rao, Q.X.; Yao, T.T.; Zhang, B.Z.; Lin, R.C.; Chen, Z.L.; Zhou, H.; Wang, L.J.; Lu, H.W.; Chen, Q.; Di, N.; et al. Expression and functional role of ALDH1 in cervical carcinoma cells. Asian Pac. J. Cancer Prev. 2012, 13, 1325–1331, doi:10.7314/apjcp.2012.13.4.1325.
- Kyo, S.; Kato, K. Endometrial Cancer Stem Cell as a Potential Therapeutic Target. Semin. Reprod. Med. 2015, 33, 341–349, doi:10.1055/s-0035-1563407.
- Van der Zee, M.; Sacchetti, A.; Cansoy, M.; Joosten, R.; Teeuwssen, M.; Heijmans-Antonissen, C.; Ewing-Graham, P.C.; Burger, C.W.; Blok, L.J.; Fodde, R. IL6/JAK1/STAT3 Signaling Blockade in Endometrial Cancer Affects the ALDHhi/CD126+ Stem-like Component and Reduces Tumor Burden. Cancer Res. 2015, 75, 3608–3622, doi:10.1158/0008-5472.CAN-14-2498.
- Rahadiani, N.; Ikeda, J.; Mamat, S.; Matsuzaki, S.; Ueda, Y.; Umehara, R.; Tian, T.; Wang, Y.; Enomoto, T.; Kimura, T.; et al. Expression of aldehyde dehydrogenase 1 (ALDH1) in endometrioid adenocarcinoma and its clinical implications. Cancer Sci. 2011, 102, 903–908, doi:10.1111/j.1349-7006.2011.01864.x.
- Kitson, S.J.; Rosser, M.; Fischer, D.P.; Marshall, K.M.; Clarke, R.B.; Crosbie, E.J. Targeting Endometrial Cancer Stem Cell Activity with Metformin Is Inhibited by Patient-Derived Adipocyte-Secreted Factors. Cancers 2019, 11, doi:10.3390/cancers11050653.
- Wang, Y.C.; Yo, Y.T.; Lee, H.Y.; Liao, Y.P.; Chao, T.K.; Su, P.H.; Lai, H.C. ALDH1-bright epithelial ovarian cancer cells are associated with CD44 expression, drug resistance, and poor clinical outcome. Am. J. Pathol. 2012, 180, 1159–1169, doi:10.1016/j.ajpath.2011.11.015.
- Pascal, L.E.; Oudes, A.J.; Petersen, T.W.; Goo, Y.A.; Walashek, L.S.; True, L.D.; Liu, A.Y. Molecular and cellular characterization of ABCG2 in the prostate. BMC Urol. 2007, 7, 6, doi:10.1186/1471-2490-7-6.
- Zong, X.; Nephew, K.P. Ovarian Cancer Stem Cells: Role in Metastasis and Opportunity for Therapeutic Targeting. Cancers 2019, 11, doi:10.3390/cancers11070934.
- Condello, S.; Morgan, C.A.; Nagdas, S.; Cao, L.; Turek, J.; Hurley, T.D.; Matei, D. beta-Catenin-regulated ALDH1A1 is a target in ovarian cancer spheroids. Oncogene 2015, 34, 2297–2308, doi:10.1038/onc.2014.178.
- Landen, C.N., Jr.; Goodman, B.; Katre, A.A.; Steg, A.D.; Nick, A.M.; Stone, R.L.; Miller, L.D.; Mejia, P.V.; Jennings, N.B.; Gershenson, D.M.; et al. Targeting aldehyde dehydrogenase cancer stem cells in ovarian cancer. Mol. Cancer Ther 2010, 9, 3186–3199, doi:10.1158/1535-7163.MCT-10-0563.
- Chefetz, I.; Grimley, E.; Yang, K.; Hong, L.; Vinogradova, E.V.; Suciu, R.; Kovalenko, I.; Karnak, D.; Morgan, C.A.; Chtcherbinine, M.; et al. A Pan-ALDH1A Inhibitor Induces Necroptosis in Ovarian Cancer Stem-like Cells. Cell Rep. 2019, 26, 3061–3075, doi:10.1016/j.celrep.2019.02.032.
- Cui, T.; Srivastava, A.K.; Han, C.; Wu, D.; Wani, N.; Liu, L.; Gao, Z.; Qu, M.; Zou, N.; Zhang, X.; et al. DDB2 represses ovarian cancer cell dedifferentiation by suppressing ALDH1A1. Cell Death Dis 2018, 9, 561, doi:10.1038/s41419-018-0585-y.
- Januchowski, R.; Wojtowicz, K.; Sterzyska, K.; Sosiska, P.; Andrzejewska, M.; Zawierucha, P.; Nowicki, M.; Zabel, M. Inhibition of ALDH1A1 activity decreases expression of drug transporters and reduces chemotherapy resistance in ovarian cancer cell lines. Int. J. Biochem. Cell Biol. 2016, 78, 248–259, doi:10.1016/j.biocel.2016.07.017.
- Wang, Y.; Cardenas, H.; Fang, F.; Condello, S.; Taverna, P.; Segar, M.; Liu, Y.; Nephew, K.P.; Matei, D. Epigenetic targeting of ovarian cancer stem cells. Cancer Res. 2014, 74, 4922–4936, doi:10.1158/0008-5472.CAN-14-1022.
- Nwani, N.G.; Condello, S.; Wang, Y.; Swetzig, W.M.; Barber, E.; Hurley, T.; Matei, D. A Novel ALDH1A1 Inhibitor Targets Cells with Stem Cell Characteristics in Ovarian Cancer. Cancers 2019, 11, doi:10.3390/cancers11040502.
- Yang, S.M.; Yasgar, A.; Miller, B.; Lal-Nag, M.; Brimacombe, K.; Hu, X.; Sun, H.; Wang, A.; Xu, X.; Nguyen, K.; et al. Discovery of NCT-501, a Potent and Selective Theophylline-Based Inhibitor of Aldehyde Dehydrogenase 1A1 (ALDH1A1). J. Med. Chem. 2015, 58, 5967–5978, doi:10.1021/acs.jmedchem.5b00577.
- Steg, A.D.; Bevis, K.S.; Katre, A.A.; Ziebarth, A.; Dobbin, Z.C.; Alvarez, R.D.; Zhang, K.; Conner, M.; Landen, C.N. Stem cell pathways contribute to clinical chemoresistance in ovarian cancer. Clin. Cancer Res. 2012, 18, 869–881, doi:10.1158/1078-0432.CCR-11-2188.
- Li, Y.; Chen, T.; Zhu, J.; Zhang, H.; Jiang, H.; Sun, H. High ALDH activity defines ovarian cancer stem-like cells with enhanced invasiveness and EMT progress which are responsible for tumor invasion. Biochem. Biophys Res. Commun. 2018, 495, 1081–1088, doi:10.1016/j.bbrc.2017.11.117.
- Wu, Q.; Shi, H.; Holm, R.; Li, X.; Trope, C.; Nesland, J.M.; Suo, Z. Aldehyde dehydrogenase-1 predicts favorable prognosis in patients with vulvar squamous cell carcinoma. Anticancer Res. 2014, 34, 859–865.