Electromyography (EMG) is a bioelectrical signal to assess electrical activity produced by skeletal muscles. In the study of muscle fatigue, the electrophysiological measurement of muscles play a crucial role in collecting electrical signals from skeletal muscles. Since EMG signals usually contain a certain amount of noise, it is essential to obtain high quality data in the early stage. In addition, people prefer a painless and comfortable method when recording muscle contraction signals. This review addresses the EMG data collection methods with good quality and less pain, their applications, and the examples of actual EMG data analysis.
- biosensor
- wearable biosensor
- electrophysiology sensor
1. Electromyogrphy
Electromyography (EMG) is a bioelectrical signal for in evaluating electrical activity produced by skeletal muscles. The EMG sensor detects the motor unit potential, which is a complex potential generated by the muscle fibers of the motor unit during spontaneous activity of muscle cells, enabling analysis of muscle activity. For muscles to work, electrical stimulation must be applied to muscle cells by nerve substances generated by a command from the central nervous system. Contraction and relaxation of muscles occur as a result of electrical stimulation, and these phenomena occur in several muscles, causing the body to move [4]. When our brain commands the muscles to contract, the central nervous system connected to the brain releases neurotransmitters and the neurons that receive these substances act to transmit electrical signals to the muscles, which make the body move. The electrical signals generated in muscle cells appear as mechanical signals, muscle contraction, and relaxation, and this transformation process is called excitation–contraction coupling [5]. The EMG sensor is a device that measures the electrical signals of muscles during this process. As an example of an EMG signal, the raw data when a muscle contracted and relaxed with a 1-kg dumbbell was measured by attaching an EMG sensor to the biceps brachii, as shown in Figure 1. When the muscle contracted, the value was larger than when the muscle was relaxed. Although the degree of muscle contraction can be visually confirmed with raw data, analysis is necessary for quantitative evaluation. EMG, which checks the degree of muscle contraction, is mainly used for medical and biomechanical research purposes[6]. For medical purposes, when movement is impossible for certain reasons, an EMG sensor is used to diagnose whether it is due to a problem in the nervous system or to damage to the muscle itself. When a muscle cannot contract or relax because of a muscle function problem, an electrical signal can be measured by EMG. If a problem occurs in the nervous system, the EMG signal cannot be measured. Also, it is possible to check the muscles that are activated during specific movements and activities, and through this, it is possible to study more efficient movements and activities. With EMG, it is also possible to analyze muscle peripheral and central fatigue during specific movements. If the contractile force of the muscle decreases due to peripheral fatigue, fatigue can be measured by increasing the raw data of the EMG signal because more electrical signals are required to maintain the same force. As the speed at which action potentials and excitations are transmitted by central fatigue slows down, the conduction velocity, which is the rate at which electrochemical impulses propagate into the nerve pathways, also slows down. Muscular fatigue is a combination of two types of fatigue, and the EMG sensor can determine the overall fatigue level [7].[1][2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50]
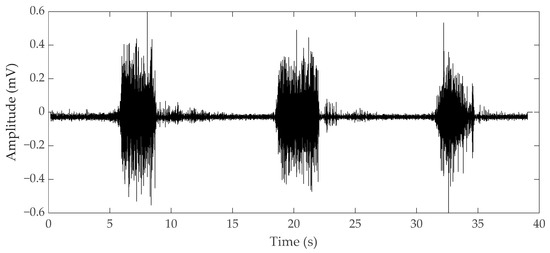
Figure 1. Electromyography (EMG) raw data during bicep brachii contraction and relaxation.
EMG appears in two forms: surface EMG (sEMG), a noninvasive measurement method, and needle EMG, an invasive method. (Figure 2) sEMG is used more widely because it has a great advantage in terms of stability. However, since needle EMG directly inserts a needle into the muscle, more accurate results can be obtained than with sEMG [8]. As a result of investigating the relationship between the trigger point and the central nervous system to find problems in muscle pain syndrome and of conducting research with EMG, the necessary EMG could not be seen with surface recording techniques [9,10,11][9][10][11]. Although there are limitations of sEMG in a specific field, the development of technology for sEMG is required for implementation in wearable sensors. Since the applicable fields differ depending on the type of EMG, we divided this section into needle and surface EMG to review the research on EMG currently underway.
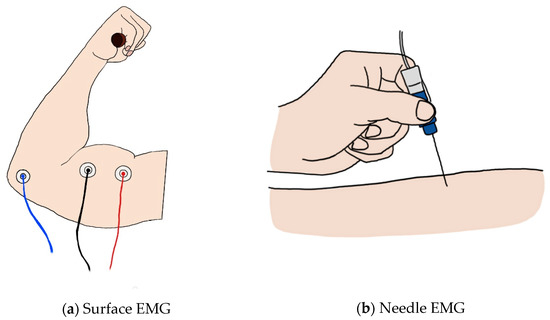
Figure 2. (a) Image of surface EMG: the red and black lines represent the + and − electrodes, and the blue line represents the ground; (b) image of the needle EMG.
1.1. Surface EMG
Surface EMG is the most widely known and used method in measuring muscle contraction. Electrical contributions made by the active motor units are measured in a noninvasive way on the skin using electrodes [7]. sEMG does not simply measure the electrical signals generated by the muscles; it represents the potential difference between two electrodes. If two electrodes are placed along muscle fibers at both ends of the motor for the muscle, the same muscle action potentials at both ends of the muscle are recorded at the same time, making it impossible to measure the potential difference. Therefore, the EMG signal is measured using a differential amplifier that rejects a common signal from both electrodes using two electrodes [12]. Since electrical signals exist even in muscles that are not in use, reference electrodes are required to exclude redundant signals. Finally, three electrodes must be attached to receive the EMG signal of one muscle. Through this process, sEMG can evaluate the contractile ability of one muscle from three signals.
In the early days of sEMG, it was used for biofeedback. For example, a study was conducted to reduce the frequency and severity of tension headaches through EMG biofeedback, and another study found that the resting level of frontalis EMG activity is higher in tension headache patients than in the general population. From this, it was argued that contraction headaches caused by constant contractions of the scalp and neck muscles could be relieved by learning to relax these muscles through biofeedback [13]. In another study, when biofeedback was given through computerized electromyographic evaluation of pelvic floor muscles, the subjective evaluation of pain decreased by 83% after 16 weeks and the resting tension level decreased by 68% [14]. However, in the above study, since only the amplitude of the simple EMG value was used to check the contraction degree of the muscle and proceeded with biofeedback, more detailed EMG parameters were studied to evaluate the muscle performance.
To obtain the parameters, EMG data are mainly analyzed in two domains: a time domain where EMG data can be viewed over time and a frequency domain that can check the frequency of EMG data within a certain range. In the time domain, the degree of muscle activation can be visually confirmed over time. Because muscles require a larger electrical signal to generate more force, they exhibit a larger amplitude in the EMG data. Therefore, it is possible to check the degree of activation for each muscle using the root mean square (RMS) value [15,16][15][16]. Fate analysis in the time domain is also possible. When a muscle continuously exerts the same force, a larger electrical signal is required to maintain the same output; therefore, the more fatigue it builds up, the greater the amplitude displayed. Fatigue can be analyzed with an increase in RMS indicating an increase in amplitude [17]. In addition, various parameters such as integrated EMG and maximum amplitude are used in the time domain [18]. In the frequency domain, fatigue is mainly analyzed. As muscle fatigue builds up, the speed of transmitting electrical signals slows down and the frequency is lowered, so that the conduction velocity slows down [19]. To capture this, fatigue is quantified by using parameters such as mean frequency, median frequency, and peak frequency in a specific range [18]. An example of EMG parameters for fatigue analysis can be seen in Figure 3.
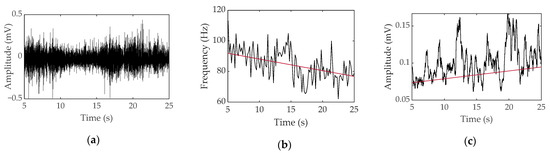
Figure 3. Surface EMG data obtained during voluntary contraction of a bicep with a 1-kg weight for a female: (a) raw data, (b) median frequency and linear regression, and (c) root mean square and linear regression.
Using these parameters, clinical studies have been conducted to measure muscle fatigue. Since the amplitude of EMG is different for each individual, fatigue was given using maximum voluntary contraction (MVC) and reliability was confirmed by analyzing the EMG parameters for fatigue (Table 1). Fatigue parameters were studied as RMS in the time domain, mean power frequency (MNF), and median power frequency (MDF) in the frequency domain, since the power spectrum frequency shift decreased. Mainly, the vastus lateralis (VL), rectus femoris (RF), vastus medialis (VM), lumbar 1–5, bicep brachii, and quadriceps were studied. Since the EMG signal has an accurate value when measured individually for one muscle rather than a complex signal of several muscles, it is limited to isometric contraction rather than dynamic contraction, which has a risk of complex measurement of several muscles. As a result of measuring EMG data during fatigue with isometric contraction, it was reported that most were reliable as indicators of fatigue, but due to the difficulty of application in dynamic contraction fatigue experiments and noise from electrode attachment, research on EMG continues.
Table 1.
Reliability of EMG parameters for fatigue indices.
| Ref | Purpose | Electrode Location | Subjects | Experimental Method | Parameters Used | Conclusion |
|---|---|---|---|---|---|---|
| [20] 2000 |
Investigation of EMG variable (MNF, RMS) for valid indicators of muscular fatigue | VL, RF, VM | 11 males, 10 females |
29]. The surface EMG for a Noninvasive Assessment of Muscles (SENIAM) project standardized the electrode attachment location and electrode size for concerted action in the Biomedical Health and Research program of the European Union [30]. However, even if the standard electrode location is used, the biggest drawback of sEMG is not compensated. The limitations from previous studies are presented in Table 2. Some obtained the same results as the isometric contraction in dynamic contraction, and some did not obtain valid data. The studies that did not obtain valid data from the isometric contraction suggested that the reason was due to the movement of various muscles rather than one muscle when performing a specific movement.
Table 2.
Clinical studies conducted by measuring EMG during movement.
| Ref | Purpose | Electrode Location | Subject | Experimental Method | Parameters Used | Conclusion | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Repetitive maximum isokinetic knee extensions | |||||||||||||
| [31] 1993 | RMS, MNF, MDF, | torque, knee joint position | MNF is a good criterion validity. | ||||||||||
| Investigation of EMG median frequency of calf muscles during an exhausting treadmill exercise | Right soleus, gastrocnemius medialis, gastrocnemius lateralis | 7 males, | 2 females |
Uphill treadmill run till the moment of exhaustion | Heartrate, ECG, median frequency, | Immediately after the run, isometric median power frequency declined. | [21] 1999 |
Investigation reliability of sEMG | |||||
| [32] 2007 |
Determination if a difference existed in the rate of fatigue of select shoulder muscles during isometric | VL, RF | shoulder elevation and if the measured rate of fatigue was consistent from day to day9 males, 9 females |
Upper trapezius, middle deltoid, serratus anterior, lower trapezius musclesIsometric knee extension | RMS, MDF, torque | MVC measurement is best suited for clinical applications from rectus femoris muscle. | |||||||
| 7 males, | 9 females | | 60% of their maximal voluntary isometric contraction force (MVIC) |
MPF | Middle deltoid appears to fatigue faster than the other shoulder muscles tested at the selected level of shoulder elevation. |
[22] 1999 |
Correlation of EMG fatigue data in the lower back to the subject’s assessment of fatigue | ||||||
| [ | L1, L5 | 33] | 25 males, 25 females |
Sørensen test | MDF, endurance time, Borg scale | The Borg scale correlated with endurance time and EMG median and mean power frequency slopes. | |||||||
| 2009 | Determination of the difference in fatigue between athletes and non-athletes | VL, VM, RF | 11 males | Maximum versus forced repetition knee extension | Blood lactate, load in forced repetition, integrated EMG | Strength athletes produced neural fatigue in high-intensity resistance exercise. | [23] 1982 |
Examination of the changes in frequency and amplitude of sEMG | Adductor pollicis, handgrip muscles, biceps, quadriceps | 6 males | MVC, fatiguing contraction (25,40,70% MVC) | RMS, center frequency | |
| [ | The center frequency of sEMG appears to be a good noninvasive index of muscle fatigue. | ||||||||||||
| 34] 1998 |
EMG assessment of back muscle function during cyclical lifting | Mind-belly of the longissimus thoracis, iliocostalis lumborum, multifidus muscles at L1, L2, L5 | 3 males, 1 female |
Dynamic and static lifting | instantaneous median frequency (Choi–Williams) | During dynamic contractions, instantaneous median frequency behavior is nonlinear and more complex than static contraction. | [24] 1979 |
Study of quantitative changes in the EMG pattern muscle fiber-type distribution | VL | 11 males | MV knee extensions | Integrated EMG, MNF | MNF decreases in FT-type muscles. |
| [35] (2005) |
Evaluation of handgrip forces using sEMG of forearm muscles | 6 forearm muscles | 8 males | Isometric gripping tasks | Grip force, normalized EMG | For standardized grips, valid predictions of grip based on EMG were produced. | [25] 1986 |
Determination of the effects of motor unit recruitment and firing frequency on the surface EMG power spectra during sustained MVC and 50% MVC of the bicep brachii muscle | Bicep brachii | 12 males | 50% MVC | ||
| [ | RMS, MNF | 36] | Increasing RMS EMG amplitude and decreasing MPF could better represent the MU activity during fatigue. | ||||||||||
| (2001) | Evaluation of the potential health effects with respect to the low back of an office chair | L3, T10 | 3 females, 7 males |
Simulated office work on a chair | Exposure variance analysis | Trunk kinematics and erector spinae EMG were strongly affected by the task performed but not by chair type. | [26] 1994 |
Examination of the relationship between EMG manifestation of fatigue and endurance time during isometric contraction of the back extensors to fatigue | Erector spine at the levels of the 10th thoracic and 3rd lumbar vertebrae | 21 males, 208 females |
Sørensen test | MF, endurance time | MFgrad is a suitable technique for monitoring back muscle fatigue. |
sEMG has a limitation in that it is a noninvasive method that is not directly inserted into the muscle. Because of the noise caused by the electrode, which is an attachment type, and due to the muscle attachment position of the electrode, it affects the conduction velocity and median frequency, parameters of EMG [27]. Standardization of the attachment method and attachment location per laboratory was required. Therefore, a study on standardizing the electrode position was conducted to obtain accurate and repeatable data on the sEMG signal parameters. Recommendations for the use and technical considerations of sEMG and a questionnaire and interpretations required for the use of sEMG were presented [6]. Several researchers studied the standardized electrode locations. For example, to provide information on the degree of uniformity of the inner zone position of 13 superficial muscles in the lower limb, an experiment was conducted and the optimal electrode placement was between the inner zone and the tendon termination according to the landmark in eight of 13 muscles [28]. This placement was suggested by examining the muscle fiber orientation and palpable bony landmarks in the abdominal muscle [
When the EMG sensor is applied to a smart device, it is possible to recognize and prevent a muscle from being injured during exercise by monitoring the movement of the muscle. Since 70 years have passed since it was used for research, if a wearable sensor is made using the results of the research conducted so far, quality of life will be greatly improved. For example, there was a study that analyzed the frequency spectrum of electrocardiography (ECG) data that can detect EMG and EEG and can differentiate sleep states divided into four with 98–99% accuracy [37]. In addition, some studies have shown that driver fatigue can be measured by ECG and EMG [38,39][38][39]. In the case of drowsy driving, ECG and EMG can be developed for wearable biosensors to detect the risk of drowsiness at one of four levels and can greatly reduce accident rates. However, since sEMG and EEG need to have electrodes attached, they are inconvenient to wear and have not yet been commercialized due to noise problems. It was mainly analyzed only in the isometric contraction of a single muscle, and since dynamic movement appears when the movements of several muscles are integrated, it is difficult to measure accurate and significant data when performing dynamic movements. Also, a wireless sensor is essential for use outside the laboratory, such as running on a track or in a playground. Currently, a wireless sEMG sensor has been developed to analyze dynamic motion, but the noise that occurs in dynamic motion has not yet been resolved. For the EMG sensor to become a wearable sensor, it is necessary to solve the problem of noise generated on the surface electrode. The solution to this will be covered in detail in the section on noise below.
1.2. Needle EMG
Unlike surface EMG, needle EMG records the electrical activity of a muscle by inserting a needle electrode directly into the muscle or by obtaining the high-frequency electrical activity generated by moving the position of the needle electrode in the muscle and an electrical signal in a rest state [40]. The measurement of needle EMG by physical stimulation causes more severe pain than that of sEMG. In order to solve this problem, a study was conducted to investigate the pain of needle EMG [41], which can be divided into two types: a concentric needle electrode and a monopolar needle electrode (Figure 4). In the former, the surrounding cannula is the reference electrode, and the latter is recorded through the surface electrode. The electrode for measuring needle EMG is mainly a concentric needle electrode that has a small recording area and can obtain a value that cancels out noise. Since needle EMG can measure accurate data, one unit of motion can be analyzed through action potentials obtained from muscle fibers that contract at a location very close to the needle electrode using triggered averaging and decomposition methods [42]. After inserting a needle to induce minimal muscle contraction, the motion unit potential is analyzed by continuously obtaining one and the same motion unit potential using amplitude triggering while gradually moving the position. Muscles are evaluated mainly by comparing the average duration and amplitude with normal values [43]. After that, multi-motor unit potentials (MUP) analysis was developed and a technique capable of simultaneously obtaining and analyzing multiple-MUP from one recording site became available and was applied [44]. The pain of needle EMG, which was caused by the movement of the existing electrode, is less than in the past with multi-MUP analysis, which is possible with one insertion. However, there are still various limitations.
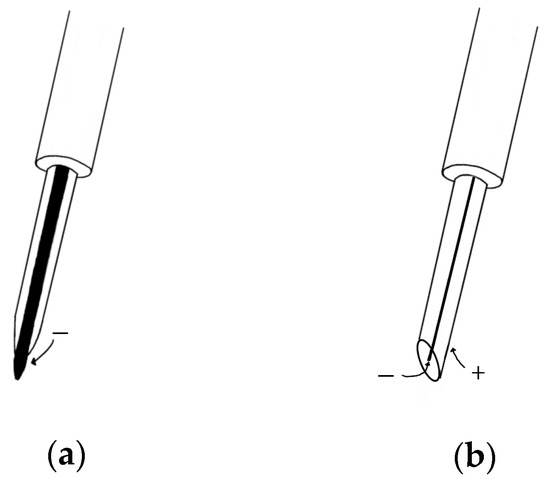
Figure 4.
(
a
) Monopolar needle electrode; (
b
) concentric needle electrode.
Electrodiagnostic physicians should conduct a needle EMG test after checking the patient’s history based on clinical data. Care should be taken regarding some patients with skin infections, skin diseases, bleeding disorders, and obesity. Since there is a risk due to various variables such as muscle location and muscle size, needle EMG requires that the placement of a needle in the longitudinal midline of the muscle is accurately inserted into the muscle of interest. Instead of these drawbacks, it can be used as a measure of the accuracy of sEMG. For example, in the vastus intermedius muscle, sEMG was used to determine if the whole muscle could be used to assess neuromuscular activation. The observation of a good correlation could be used to assess total neuromuscular activation of the VL muscle during isometric contraction at low force levels [45]. There is also a paper on the feature extraction of a forearm EMG signal for prosthetics. EMG data analysis was conducted using both needle and sEMG for recording accuracy during hand movement [46]. For analysis of neuromuscular jitter that can be measured only in a single fiber, only needle EMG can be used [47][48][49] [47,48,49] (Figure 5). EMG, which can obtain sensitive signals, is used for physiological analysis and diagnosis in a single motor unit during muscle contraction. Needle EMG is known to have a more accurate value than sEMG and is being used and studied. Needles have little noise, but considering the risk and difficulty of including them in a wearable sensor, it cannot be applied to the future development of biosensors. However, if the surface electrode is made in a way that reduces noise by bringing the reference electrode closer like a needle EMG to make it more accurate and easy to wear, it can be used in a wider range.
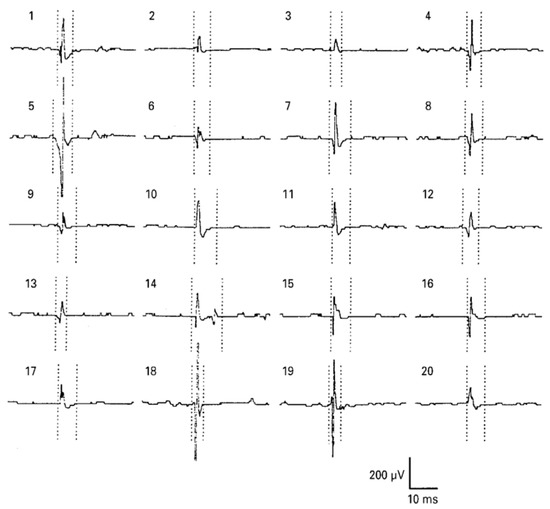
그림 5. 혀의 바늘 EMG : 건강한 피험자의 오른쪽 genioglossus 근육에서 모터 단위 활동 전위 (MUAP). MUAP 기간에 대한 마커는 5 개의 평균 MUAP 중 가장 오염되지 않은 것으로 설정되었습니다. BMJ Publishing Group Ltd.의 허가를 받아 [ 50 ] 에서 복제 됨 .Figure 5.
Needle EMG of the tongue: motor unit action potential (MUAP) from the right genioglossus muscle of a healthy subject. Markers for the MUAP duration were set on the most uncontaminated of the five averaged MUAPs. Reproduced from [50] with permission from BMJ Publishing Group Ltd.
References
- Burns, A.; Greene, B.R.; McGrath, M.J.; O'Shea, T.J.; Kuris, B.; Ayer, S.M.; Stroiescu, F.; Cionca, V. SHIMMER™–A wireless sensor platform for noninvasive biomedical research. IEEE Sensors Journal 2010, 10, 1527-1534.
- Bonato, P. Advances in wearable technology and applications in physical medicine and rehabilitation. BioMed Central: 2005.
- Reisner, A.; Shaltis, P.; McCombie, D.; Asada, H. A critical appraisal of opportunities for wearable medical sensors. In Proceedings of the 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Francisco, CA, USA, 1–5 September 2004; pp. 2149–2152.
- Ebashi, S.; Endo, M.; Ohtsuki, I. Control of muscle contraction. Quarterly reviews of biophysics 1969, 2, 351-384.
- Sandow, A. Excitation-contraction coupling in muscular response. The Yale journal of biology and medicine 1952, 25, 176–201.
- De Luca, C.J. The use of surface electromyography in biomechanics. Journal of applied biomechanics 1997, 13, 135-163.
- Farina, D.; Merletti, R.; Enoka, R.M. The extraction of neural strategies from the surface EMG: an update. Journal of Applied Physiology 2014, 117, 1215-1230.
- Merletti, R.; Parker, P.J. Electromyography: Physiology, Engineering, and Non-Invasive Applications; John Wiley & Sons: Ho-boken, NJ, USA, 2004; Volume 11.
- Travell, J.G. Myofascial trigger points: clinical view. Advances in pain research and therapy 1976, 1, 919-926.
- Chen, J.-T.; Chen, S.-M.; Kuan, T.-S.; Chung, K.-C.; Hong, C.-Z. Phentolamine effect on the spontaneous electrical activity of active loci in a myofascial trigger spot of rabbit skeletal muscle. Archives of physical medicine and rehabilitation 1998, 79, 790-794.
- Simons, D.G.; Dexter, J.R. Comparison of local twitch responses elicited by palpitation and needling of myofascial trigger points. Journal of Musculoskeletal Pain 1995, 3, 49-61.
- Campbell, W.W. Essentials of Electrodiagnostic Medicine; Demos Medical Publishing: New York, NY, USA, 2013.
- Bunzynski, T.H.; Stoyva, J.M.; Adler, C.S.; Mullaney, D.J. EMG biofeedback and tension headache: a controlled outcome study. Psychosomatic Medicine 1973.
- Glazer, H.I.; Rodke, G.; Swencionis, C.; Hertz, R.; Young, A.W. Treatment of vulvar vestibulitis syndrome with electromyographic biofeedback of pelvic floor musculature. Obstetrical & Gynecological Survey 1995, 50, 658-659.
- Milner‐Brown, H.; Stein, R. The relation between the surface electromyogram and muscular force. The Journal of physiology 1975, 246, 549-569.
- Mathiassen, S.; Winkel, J.; Hägg, G. Normalization of surface EMG amplitude from the upper trapezius muscle in ergonomic studies—a review. Journal of electromyography and kinesiology 1995, 5, 197-226.
- De Luca, C.J. Myoelectrical manifestations of localized muscular fatigue in humans. Critical reviews in biomedical engineering 1984, 11, 251.
- Nazmi, N.; Abdul Rahman, M.A.; Yamamoto, S.-I.; Ahmad, S.A.; Zamzuri, H.; Mazlan, S.A. A review of classification techniques of EMG signals during isotonic and isometric contractions. Sensors 2016, 16, 1304.
- Naeije, M.; Zorn, H. Relation between EMG power spectrum shifts and muscle fibre action potential conduction velocity changes during local muscular fatigue in man. European journal of applied physiology and occupational physiology 1982, 50, 23-33.
- Gerdle, B.; Larsson, B.; Karlsson, S. Criterion validation of surface EMG variables as fatigue indicators using peak torque: a study of repetitive maximum isokinetic knee extensions. Journal of Electromyography and Kinesiology 2000, 10, 225-232.
- Kollmitzer, J.; Ebenbichler, G.R.; Kopf, A. Reliability of surface electromyographic measurements. Clinical Neurophysiology 1999, 110, 725-734.
- Dedering, Å.; Németh, G.; Harms-Ringdahl, K. Correlation between electromyographic spectral changes and subjective assessment of lumbar muscle fatigue in subjects without pain from the lower back. Clinical Biomechanics 1999, 14, 103-111.
- Petrofsky, J.S.; Glaser, R.M.; Phillips, C.A.; Lind, A.R.; Williams, C. Evaluation of the amplitude and frequency components of the surface EMG as an index of muscle fatigue. Ergonomics 1982, 25, 213-223.
- Komi, P.V.; Tesch, P. EMG frequency spectrum, muscle structure, and fatigue during dynamic contractions in man. European journal of applied physiology and occupational physiology 1979, 42, 41-50.
- Moritani, T.; Muro, M.; Nagata, A. Intramuscular and surface electromyogram changes during muscle fatigue. Journal of Applied Physiology 1986, 60, 1179-1185.
- Mannion, A.F.; Dolan, P. Electromyographic median frequency changes during isometric contraction of the back extensors to fatigue. Spine 1994, 19, 1223-1229.
- Roy, S.H.; De Luca, C.J.; Schneider, J. Effects of electrode location on myoelectric conduction velocity and median frequency estimates. Journal of applied physiology 1986, 61, 1510-1517.
- Rainoldi, A.; Melchiorri, G.; Caruso, I. A method for positioning electrodes during surface EMG recordings in lower limb muscles. Journal of neuroscience methods 2004, 134, 37-43.
- Ng, J.; Kippers, V.; Richardson, C. Muscle fibre orientation of abdominal muscles and suggested surface EMG electrode positions. Electromyography and clinical neurophysiology 1998, 38, 51-58.
- Hermens, H.J.; Freriks, B.; Merletti, R.; Stegeman, D.; Blok, J.; Rau, G.; Disselhorst-Klug, C.; Hägg, G. European recommendations for surface electromyography. Roessingh research and development 1999, 8, 13-54.
- Ament, W.; Bonga, G.J.; Hof, A.L.; Verkerke, G.J. EMG median power frequency in an exhausting exercise. Journal of electromyography and kinesiology 1993, 3, 214-220.
- Minning, S.; Eliot, C.A.; Uhl, T.L.; Malone, T.R. EMG analysis of shoulder muscle fatigue during resisted isometric shoulder elevation. Journal of Electromyography and Kinesiology 2007, 17, 153-159.
- Dimitrov, G.V.; Arabadzhiev, T.I.; Mileva, K.N.; Bowtell, J.L.; Crichton, N.; Dimitrova, N.A. Muscle fatigue during dynamic contractions assessed by new spectral indices. Medicine and science in sports and exercise 2006, 38, 1971.
- Roy, S.H.; Bonato, P.; Knaflitz, M. EMG assessment of back muscle function during cyclical lifting. Journal of Electromyography and Kinesiology 1998, 8, 233-245.
- Hoozemans, M.J.; Van Dieen, J.H. Prediction of handgrip forces using surface EMG of forearm muscles. Journal of electromyography and kinesiology 2005, 15, 358-366.
- Van Dieën, J.; De Looze, M.; Hermans, V. Effects of dynamic office chairs on trunk kinematics, trunk extensor EMG and spinal shrinkage. Ergonomics 2001, 44, 739-750.
- Akin, M.; Kurt, M.B.; Sezgin, N.; Bayram, M. Estimating vigilance level by using EEG and EMG signals. Neural Computing and Applications 2008, 17, 227-236.
- Lal, S.K.; Craig, A. Driver fatigue: electroencephalography and psychological assessment. Psychophysiology 2002, 39, 313-321.
- Fu, R.; Wang, H. Detection of driving fatigue by using noncontact EMG and ECG signals measurement system. International journal of neural systems 2014, 24, 1450006.
- Rubin, D.I. Needle electromyography: basic concepts and patterns of abnormalities. Neurologic clinics 2012, 30, 429-456.
- Strommen, J.A.; Daube, J.R. Determinants of pain in needle electromyography. Clinical neurophysiology 2001, 112, 1414-1418.
- Boe, S.G.; Stashuk, D.W.; Doherty, T.J. Motor unit number estimation by decomposition‐enhanced spike‐triggered averaging: Control data, test–retest reliability, and contractile level effects. Muscle & Nerve: Official Journal of the American Association of Electrodiagnostic Medicine 2004, 29, 693-699.
- Nandedkar, S.D.; Barkhaus, P.E.; Sanders, D.B.; Stålberg, E.V. Analysis of amplitude and area of concentric needle EMG motor unit action potentials. Electroencephalography and clinical neurophysiology 1988, 69, 561-567.
- Stålberg, E.; Falck, B.; Sonoo, M.; Stålberg, S.; Åström, M. Multi-MUP EMG analysis—a two year experience in daily clinical work. Electroencephalography and Clinical Neurophysiology/Electromyography and Motor Control 1995, 97, 145-154.
- Watanabe, K.; Akima, H. Validity of surface electromyography for vastus intermedius muscle assessed by needle electromyography. Journal of Neuroscience Methods 2011, 198, 332-335.
- Rafiee, J.; Rafiee, M.; Yavari, F.; Schoen, M. Feature extraction of forearm EMG signals for prosthetics. Expert Systems with Applications 2011, 38, 4058-4067.
- Stålberg, E. Jitter analysis with concentric needle electrodes. Annals of the New York Academy of Sciences 2012, 1274, 77-85.
- Ertaş, M.; Baslo, M.B.; Yildiz, N.; Yazici, J.; Öge, A.E. Concentric needle electrode for neuromuscular jitter analysis. Muscle & Nerve: Official Journal of the American Association of Electrodiagnostic Medicine 2000, 23, 715-719.
- Sarrigiannis, P.G.; Kennett, R.P.; Read, S.; Farrugia, M.E. Single‐fiber EMG with a concentric needle electrode: validation in myasthenia gravis. Muscle & Nerve: Official Journal of the American Association of Electrodiagnostic Medicine 2006, 33, 61-65.
- Finsterer, J.; Fuglsang-Frederiksen, A.; Mamoli, B. Needle EMG of the tongue: motor unit action potential versus peak ratio analysis in limb and bulbar onset amyotrophic lateral sclerosis. Journal of Neurology, Neurosurgery & Psychiatry 1997, 63, 175-180.
